Subgrading of G2 Pancreatic Neuroendocrine Tumors as 2A (Ki67 3% to < 10%) Versus 2B (10% to ≤ 20%) Identifies Behaviorally Distinct Subsets in Keeping with the Evolving Management Protocols
- PMID: 38955993
- PMCID: PMC11413052
- DOI: 10.1245/s10434-024-15632-y
Subgrading of G2 Pancreatic Neuroendocrine Tumors as 2A (Ki67 3% to < 10%) Versus 2B (10% to ≤ 20%) Identifies Behaviorally Distinct Subsets in Keeping with the Evolving Management Protocols
Abstract
Background: Grade 1/2 PanNETs are mostly managed similarly, typically without any adjunct treatment with the belief that their overall metastasis rate is low. In oncology literature, Ki67-index of 10% is increasingly being used as the cutoff in stratifying patients to different protocols, although there are no systematic pathology-based studies supporting this approach.
Methods: Ki67-index was correlated with clinicopathologic parameters in 190 resected PanNETs. A validation cohort (n = 145) was separately analyzed.
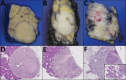
Results: In initial cohort, maximally selected rank statistics method revealed 12% to be the discriminatory cutoff (close to 10% rule of thumb). G2b cases had liver/distant metastasis rate of almost threefold higher than that of G2a and showed significantly higher frequency of all histopathologic signs of aggressiveness (tumor size, perineural/vascular invasion, infiltrative growth pattern, lymph node metastasis). In validation cohort, these figures were as striking. When all cases were analyzed together, compared with G1, the G2b category had nine times higher liver/distant metastasis rate (6.1 vs. 58.5%; p < 0.001) and three times higher lymph node metastasis rate (20.5 vs. 65.1%; p < 0.001).
Conclusions: G2b PanNETs act very similar to G3, supporting management protocols that regard them as potential therapy candidates. Concerning local management, metastatic behavior in G2b cases indicate they may not be as amenable for conservative approaches, such as watchful waiting or enucleation. This substaging should be considered into diagnostic guidelines, and clinical trials need to be devised to determine the more appropriate management protocols for G2b (10% to ≤ 20%) group, which shows liver/distant metastasis in more than half of the cases, which at minimum warrants closer follow-up.
Keywords: Grade; Ki-67 proliferative index; Metastasis; Pancreatic neuroendocrine tumor.
© 2024. The Author(s).
Conflict of interest statement
Authors declare that they have no conflict of interest to disclose.
Figures
References
-
- Anderson CW, Bennett JJ. Clinical presentation and diagnosis of pancreatic neuroendocrine tumors. Surg Oncol Clin N Am. 2016;25(2):363–74. 10.1016/j.soc.2015.12.003. - PubMed
-
- Guilmette JM, Nosé V. Neoplasms of the neuroendocrine pancreas: an update in the classification, definition, and molecular genetic advances. Adv Anat Pathol. 2019;26(1):13–30. 10.1097/pap.0000000000000201. - PubMed
-
- Mintziras I, Keck T, Werner J, et al. Implementation of current ENETS guidelines for surgery of small (≤ 2 cm) pancreatic neuroendocrine neoplasms in the German surgical community: an analysis of the prospective DGAV StuDoQ|Pancreas registry. World J Surg. 2019;43(1):175–82. 10.1007/s00268-018-4751-2. - PubMed
-
- Basturk O, Yang Z, Tang LH, et al. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol. 2015;39(5):683–90. 10.1097/PAS.0000000000000408. - PMC - PubMed
-
- Umetsu SE, Kakar S, Basturk O, et al. Integrated genomic and clinicopathologic approach distinguishes pancreatic grade 3 neuroendocrine tumor from neuroendocrine carcinoma and identifies a subset with molecular overlap. Mod Pathol. 2023;36(3):100065. 10.1016/j.modpat.2022.100065. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical