RSL3 enhances ROS-mediated cell apoptosis of myelodysplastic syndrome cells through MYB/Bcl-2 signaling pathway
- PMID: 38956026
- PMCID: PMC11219730
- DOI: 10.1038/s41419-024-06866-5
RSL3 enhances ROS-mediated cell apoptosis of myelodysplastic syndrome cells through MYB/Bcl-2 signaling pathway
Abstract
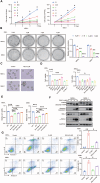
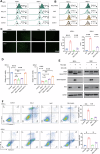
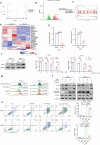
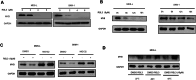
Myelodysplastic syndromes (MDS) are clonal hematopoietic malignancies and seriously threaten people's health. Current therapies include bone marrow transplantation and several hypomethylating agents. However, many elderly patients cannot benefit from bone marrow transplantation and many patients develop drug resistance to hypomethylating agents, making it urgent to explore novel therapy. RSL3 can effectively induce ferroptosis in various tumors and combination of RSL3 and hypomethylating agents is promising to treat many tumors. However, its effect in MDS was unknown. In this study, we found that RSL3 inhibited MDS cell proliferation through inducing ROS-dependent apoptosis. RSL3 inhibited Bcl-2 expression and increased caspase 3 and PARP cleavage. RNA-seq analysis revealed that MYB may be a potential target of RSL3. Rescue experiments showed that overexpression of MYB can rescue MDS cell proliferation inhibition caused by RSL3. Cellular thermal shift assay showed that RSL3 binds to MYB to exert its function. Furthermore, RSL3 inhibited tumor growth and decreased MYB and Bcl-2 expression in vivo. More importantly, RSL3 decreased the viability of bone marrow mononuclear cells (BMMCs) isolated from MDS patients, and RSL3 had a synergistic effect with DAC in MDS cells. Our studies have uncovered RSL3 as a promising compound and MYB/Bcl-2 signaling pathway as a potential target for MDS treatment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

