A chemogenetic approach for dopamine imaging with tunable sensitivity
- PMID: 38956067
- PMCID: PMC11219860
- DOI: 10.1038/s41467-024-49442-3
A chemogenetic approach for dopamine imaging with tunable sensitivity
Abstract
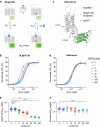
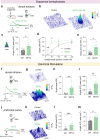
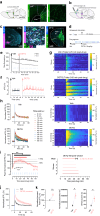
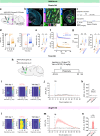
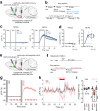
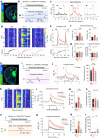
Genetically-encoded dopamine (DA) sensors enable high-resolution imaging of DA release, but their ability to detect a wide range of extracellular DA levels, especially tonic versus phasic DA release, is limited by their intrinsic affinity. Here we show that a human-selective dopamine receptor positive allosteric modulator (PAM) can be used to boost sensor affinity on-demand. The PAM enhances DA detection sensitivity across experimental preparations (in vitro, ex vivo and in vivo) via one-photon or two-photon imaging. In vivo photometry-based detection of optogenetically-evoked DA release revealed that DETQ administration produces a stable 31 minutes window of potentiation without effects on animal behavior. The use of the PAM revealed region-specific and metabolic state-dependent differences in tonic DA levels and enhanced single-trial detection of behavior-evoked phasic DA release in cortex and striatum. Our chemogenetic strategy can potently and flexibly tune DA imaging sensitivity and reveal multi-modal (tonic/phasic) DA signaling across preparations and imaging approaches.
© 2024. The Author(s).
Conflict of interest statement
T.P. is a co-inventor on a patent application (PCT/US17/62993) related to the genetically encoded sensor technology described in this article. Xin Zhou, J.K. and K.A.S. are employees of Eli Lilly and company. All other authors have no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
- 310030_196455/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- 101016787/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- F32 DA051135/DA/NIDA NIH HHS/United States
- 310030L_212508/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- R01 DA035821/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

