Design and radiosynthesis of class-IIa HDAC inhibitor with high molar activity via repositioning the 18F-radiolabel
- PMID: 38956204
- PMCID: PMC11219833
- DOI: 10.1038/s41598-024-65668-z
Design and radiosynthesis of class-IIa HDAC inhibitor with high molar activity via repositioning the 18F-radiolabel
Abstract
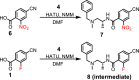
The design and radiosynthesis of [18F]NT376, a high potency inhibitor of class-IIa histone deacetylases (HDAC) is reported. We utilized a three-step radiochemical approach that led to the radiosynthesis of [18F]NT376 in a good radiochemical yield, (17.0 ± 3%, decay corrected), high radiochemical purity (> 97%) and relatively high molar activity of 185.0 GBq/µmol (> 5.0 Ci/µmol). The repositioning of the 18F-radiolabel into a phenyl ring (18F-Fluoro-aryl) of the class-IIa HDAC inhibitor avoided the shortcomings of the direct radiolabeling of the 5-trifluoromethyl-1,2,4-oxadiazole moiety that was reported by us previously and was associated with low molar activity (0.74-1.51 GBq/µmol, 20-41 mCi/µmol). This radiochemical approach could find a wider application for radiolabeling similar molecules with good radiochemical yield and high molar activity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

