High-dimensional single-cell analysis of human natural killer cell heterogeneity
- PMID: 38956378
- PMCID: PMC11291291
- DOI: 10.1038/s41590-024-01883-0
High-dimensional single-cell analysis of human natural killer cell heterogeneity
Abstract
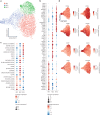
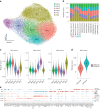
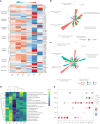
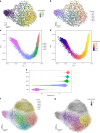
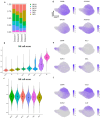
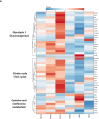
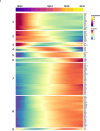
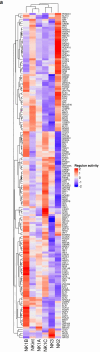
Natural killer (NK) cells are innate lymphoid cells (ILCs) contributing to immune responses to microbes and tumors. Historically, their classification hinged on a limited array of surface protein markers. Here, we used single-cell RNA sequencing (scRNA-seq) and cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq) to dissect the heterogeneity of NK cells. We identified three prominent NK cell subsets in healthy human blood: NK1, NK2 and NK3, further differentiated into six distinct subgroups. Our findings delineate the molecular characteristics, key transcription factors, biological functions, metabolic traits and cytokine responses of each subgroup. These data also suggest two separate ontogenetic origins for NK cells, leading to divergent transcriptional trajectories. Furthermore, we analyzed the distribution of NK cell subsets in the lung, tonsils and intraepithelial lymphocytes isolated from healthy individuals and in 22 tumor types. This standardized terminology aims at fostering clarity and consistency in future research, thereby improving cross-study comparisons.
© 2024. The Author(s).
Conflict of interest statement
E.V. and C.V. are employees of Innate Pharma. K.-J.M. is a consultant at Fate Therapeutics and Vycellix and receives research support from Fate Therapeutics, Oncopeptides for studies unrelated to this work. The other authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

