Vitamin K: a potential missing link in critical illness-a scoping review
- PMID: 38956732
- PMCID: PMC11218309
- DOI: 10.1186/s13054-024-05001-2
Vitamin K: a potential missing link in critical illness-a scoping review
Abstract
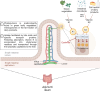
Background: Vitamin K is essential for numerous physiological processes, including coagulation, bone metabolism, tissue calcification, and antioxidant activity. Deficiency, prevalent in critically ill ICU patients, impacts coagulation and increases the risk of bleeding and other complications. This review aims to elucidate the metabolism of vitamin K in the context of critical illness and identify a potential therapeutic approach.
Methods: In December 2023, a scoping review was conducted using the PRISMA Extension for Scoping Reviews. Literature was searched in PubMed, Embase, and Cochrane databases without restrictions. Inclusion criteria were studies on adult ICU patients discussing vitamin K deficiency and/or supplementation.
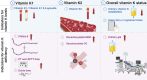
Results: A total of 1712 articles were screened, and 13 met the inclusion criteria. Vitamin K deficiency in ICU patients is linked to malnutrition, impaired absorption, antibiotic use, increased turnover, and genetic factors. Observational studies show higher PIVKA-II levels in ICU patients, indicating reduced vitamin K status. Risk factors include inadequate intake, disrupted absorption, and increased physiological demands. Supplementation studies suggest vitamin K can improve status but not normalize it completely. Vitamin K deficiency may correlate with prolonged ICU stays, mechanical ventilation, and increased mortality. Factors such as genetic polymorphisms and disrupted microbiomes also contribute to deficiency, underscoring the need for individualized nutritional strategies and further research on optimal supplementation dosages and administration routes.
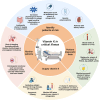
Conclusions: Addressing vitamin K deficiency in ICU patients is crucial for mitigating risks associated with critical illness, yet optimal management strategies require further investigation.
Impact research: To the best of our knowledge, this review is the first to address the prevalence and progression of vitamin K deficiency in critically ill patients. It guides clinicians in diagnosing and managing vitamin K deficiency in intensive care and suggests practical strategies for supplementing vitamin K in critically ill patients. This review provides a comprehensive overview of the existing literature, and serves as a valuable resource for clinicians, researchers, and policymakers in critical care medicine.
Keywords: Gla protein; ICU; Micronutrients; PIVKA-II; Vitamin K.
© 2024. The Author(s).
Conflict of interest statement
Prof. Dr. Van Zanten reported receiving honoraria for advisory board meetings, lectures, research, and travel expenses from AOP Pharma, Abbott, Baxter, Cardinal Health, Danone-Nutricia, DIM3, Fresenius Kabi, GE Healthcare, InBody, Mermaid, Rousselot, and Lyric. The other authors have nothing to declare.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

