Optimized rAAV8 targeting acinar KLF4 ameliorates fibrosis in chronic pancreatitis via exosomes-enriched let-7s suppressing pancreatic stellate cells activation
- PMID: 38956871
- PMCID: PMC11405174
- DOI: 10.1016/j.ymthe.2024.06.030
Optimized rAAV8 targeting acinar KLF4 ameliorates fibrosis in chronic pancreatitis via exosomes-enriched let-7s suppressing pancreatic stellate cells activation
Abstract
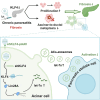
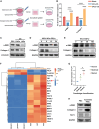
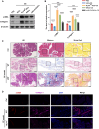
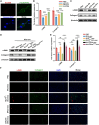
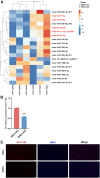
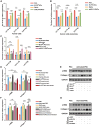
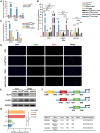
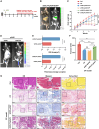
Chronic pancreatitis (CP) is marked by progressive fibrosis and the activation of pancreatic stellate cells (PSCs), accompanied by the destruction of pancreatic parenchyma, leading to the loss of acinar cells (ACs). Few research studies have explored the mechanism by which damaged ACs (DACs) contribute to PSCs activation and pancreatic fibrosis. Currently, there are no effective drugs for curing CP or limiting the progression of pancreatic fibrosis. In this research, co-culture with intact acinar cells (IACs) suppressed PSC activation, while co-culture with DACs did the opposite. Krüppel-like factor 4 (KLF4) was significantly upregulated in DACs and was established as the key molecule that switches ACs from PSCs-suppressor to PSCs-activator. We revealed the exosomes of IACs contributed to the anti-activated function of IACs-CS on PSCs. MiRNome profiling showed that let-7 family is significantly enriched in IAC-derived exosomes (>30% miRNome), which partially mediates IACs' suppressive impacts on PSCs. Furthermore, it has been observed that the enrichment of let-7 in exosomes was influenced by the expression level of KLF4. Mechanistic studies demonstrated that KLF4 in ACs upregulated Lin28A, thereby decreasing let-7 levels in AC-derived exosomes, and thus promoting PSCs activation. We utilized an adeno-associated virus specifically targeting KLF4 in ACs (shKLF4-pAAV) to suppress PSCs activation in CP, resulting in reduced pancreatic fibrosis. IAC-derived exosomes hold potential as potent weapons against PSCs activation via let-7s, while activated KLF4/Lin28A signaling in DACs diminished such functions. ShKLF4-pAAV holds promise as a novel therapeutic approach for CP.
Keywords: AAV; KLF4; Lin28A; MiRNome; chronic pancreatitis; exosome; fibrosis; let-7.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
Similar articles
-
Notch3 enhances the synergistic effect of all-trans retinoic acid and calcipotriol in pancreatic stellate cell activation.J Transl Med. 2025 Jun 22;23(1):694. doi: 10.1186/s12967-025-06666-1. J Transl Med. 2025. PMID: 40545532 Free PMC article.
-
Calpain Inhibitor Calpeptin Improves Pancreatic Fibrosis in Mice with Chronic Pancreatitis by Inhibiting the Activation of Pancreatic Stellate Cells.Curr Mol Pharmacol. 2024;17:e18761429241425. doi: 10.2174/0118761429241425231107044453. Curr Mol Pharmacol. 2024. PMID: 38258594
-
COMP promotes pancreatic fibrosis by activating pancreatic stellate cells through CD36-ERK/AKT signaling pathways.Cell Signal. 2024 Jun;118:111135. doi: 10.1016/j.cellsig.2024.111135. Epub 2024 Mar 11. Cell Signal. 2024. PMID: 38479555
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Trypsin in pancreatitis: The culprit, a mediator, or epiphenomenon?World J Gastroenterol. 2024 Nov 7;30(41):4417-4438. doi: 10.3748/wjg.v30.i41.4417. World J Gastroenterol. 2024. PMID: 39534420 Free PMC article. Review.
Cited by
-
Harnessing extracellular vesicles for pancreatic fibrosis therapy.Mol Ther. 2024 Aug 7;32(8):2441-2443. doi: 10.1016/j.ymthe.2024.07.004. Epub 2024 Jul 20. Mol Ther. 2024. PMID: 39033752 Free PMC article. No abstract available.
-
Notch3 enhances the synergistic effect of all-trans retinoic acid and calcipotriol in pancreatic stellate cell activation.J Transl Med. 2025 Jun 22;23(1):694. doi: 10.1186/s12967-025-06666-1. J Transl Med. 2025. PMID: 40545532 Free PMC article.
-
Exosomal miRNAs in pancreatitis: Mechanisms and potential applications (Review).Mol Med Rep. 2025 Aug;32(2):210. doi: 10.3892/mmr.2025.13575. Epub 2025 May 26. Mol Med Rep. 2025. PMID: 40417921 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

