Angiotensin-Converting Enzyme Inhibitors and Other Medications Associated With Angioedema
- PMID: 38957198
- PMCID: PMC11218608
- DOI: 10.7759/cureus.49306
Angiotensin-Converting Enzyme Inhibitors and Other Medications Associated With Angioedema
Abstract
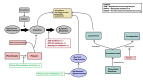
Angioedema is a localized swelling of the dermis, subcutaneous tissues, and/or submucosal tissues caused by fluid extravasation into these tissues. Angioedema is associated with certain vasoactive molecules and is typically mediated by histamine or bradykinin. It manifests clinically as facial edema, swelling of the extremities and urogenital area, and potential involvement of the larynx, leading to dyspnea and inspiratory stridor, which can become life-threatening. Histamine-mediated angioedema is associated with urticaria and pruritus and will show classic signs of allergic (type 1 hypersensitivity) reactions. Bradykinin-mediated angioedema is often familial (hereditary angioedema) and is more often associated with gastrointestinal symptoms (abdominal pain, nausea, vomiting, diarrhea), edema of the extremities and trunk, and a lack of urticaria and pruritus. Angiotensin-converting enzyme inhibitors (ACEIs) are a class of medications commonly prescribed for hypertension, heart failure, and diabetic nephropathy. ACEIs are associated with an increased risk of angioedema, which can range from a mild reaction to severe and life-threatening. ACEI-induced angioedema is a bradykinin-mediated reaction that can occur in individuals with a genetic predisposition. Other medications, such as angiotensin receptor blockers, nonsteroidal anti-inflammatory drugs, and certain antibiotics, most notably those in the beta-lactam class, can also cause drug-induced angioedema. The present investigation describes current knowledge of the pathophysiology, epidemiology, clinical manifestations, predisposing factors, and management of drug-induced angioedema.
Keywords: ace inhibitor; adverse drug reactions; angioedema; bradykinin; drug-induced angioedema; nonsteroidal anti-inflammatory drugs (nsaids); pathophysiology.
Copyright © 2023, Landry et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Angiotensin-converting enzyme inhibitor and other drug-associated angioedema. Stone C, Brown NJ. https://pubmed.ncbi.nlm.nih.gov/28687104/ Immunol Allergy Clin North Am. 2017;37:483–495. - PubMed
-
- Multinational experience with hypersensitivity drug reactions in Latin America. Jares EJ, Sánchez-Borges M, Cardona-Villa R, et al. Ann Allergy Asthma Immunol. 2014;113:282–289. - PubMed
-
- Genetic variants of the arachidonic acid pathway in non-steroidal anti-inflammatory drug-induced acute urticaria. Cornejo-García JA, Jagemann LR, Blanca-López N, et al. Clin Exp Allergy. 2012;42:1772–1781. - PubMed
-
- The burden of hospitalizations and emergency department visits with hereditary angioedema and angioedema in the United States, 2007. Zilberberg MD, Jacobsen T, Tillotson G. Allergy Asthma Proc. 2010;31:511–519. - PubMed
-
- The burden of hospitalizations and emergency department visits with hereditary angioedema and angioedema in the United States. Zilberberg MD, Jacobsen T, Tillotson G. Allergy Asthma Proc. 20072010;31:511–519. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous