An innovative intramedullary bone graft harvesting concept as a fundamental component of scaffold-guided bone regeneration: A preclinical in vivo validation
- PMID: 38957270
- PMCID: PMC11215842
- DOI: 10.1016/j.jot.2024.05.002
An innovative intramedullary bone graft harvesting concept as a fundamental component of scaffold-guided bone regeneration: A preclinical in vivo validation
Abstract
Background: The deployment of bone grafts (BGs) is critical to the success of scaffold-guided bone regeneration (SGBR) of large bone defects. It is thus critical to provide harvesting devices that maximize osteogenic capacity of the autograft while also minimizing graft damage during collection. As an alternative to the Reamer-Irrigator-Aspirator 2 (RIA 2) system - the gold standard for large-volume graft harvesting used in orthopaedic clinics today - a novel intramedullary BG harvesting concept has been preclinically introduced and referred to as the ARA (aspirator + reaming-aspiration) concept. The ARA concept uses aspiration of the intramedullary content, followed by medullary reaming-aspiration of the endosteal bone. This concept allows greater customization of BG harvesting conditions vis-à-vis the RIA 2 system. Following its successful in vitro validation, we hypothesized that an ARA concept-collected BG would have comparable in vivo osteogenic capacity compared to the RIA 2 system-collected BG.
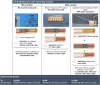
Methods: We used 3D-printed, medical-grade polycaprolactone-hydroxyapatite (mPCL-HA, wt 96 %:4 %) scaffolds with a Voronoi design, loaded with or without different sheep-harvested BGs and tested them in an ectopic bone formation rat model for up to 8 weeks.
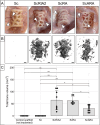
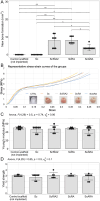
Results: Active bone regeneration was observed throughout the scaffold-BG constructs, particularly on the surface of the bone chips with endochondral bone formation, and highly vascularized tissue formed within the fully interconnected pore architecture. There were no differences between the BGs derived from the RIA 2 system and the ARA concept in new bone volume formation and in compression tests (Young's modulus, p = 0.74; yield strength, p = 0.50). These results highlight that the osteogenic capacities of the mPCL-HA Voronoi scaffold loaded with BGs from the ARA concept and the RIA 2 system are equivalent.
Conclusion: In conclusion, the ARA concept offers a promising alternative to the RIA 2 system for harvesting BGs to be clinically integrated into SGBR strategies.
The translational potential of this article: Our results show that biodegradable composite scaffolds loaded with BGs from the novel intramedullary harvesting concept and the RIA 2 system have equivalent osteogenic capacity. Thus, the innovative, highly intuitive intramedullary harvesting concept offers a promising alternative to the RIA 2 system for harvesting bone grafts, which are an important component for the routine translation of SGBR concepts into clinical practice.
Keywords: Bone graft; Bone regeneration; Intramedullary harvesting; Polycaprolactone; Scaffold; Voronoi.
© 2024 The Authors.
Figures




Similar articles
-
Assessing Cardiopulmonary Safety of Intramedullary Bone Graft Harvesting: A Comparative Study of the RIA 2 System and the ARA Concept.J Orthop Res. 2025 May;43(5):984-993. doi: 10.1002/jor.26059. Epub 2025 Feb 25. J Orthop Res. 2025. PMID: 40001236 Free PMC article.
-
An in vivo study to investigate an original intramedullary bone graft harvesting technology.Eur J Med Res. 2023 Sep 15;28(1):349. doi: 10.1186/s40001-023-01328-8. Eur J Med Res. 2023. PMID: 37715198 Free PMC article.
-
Clinical translation of a patient-specific scaffold-guided bone regeneration concept in four cases with large long bone defects.J Orthop Translat. 2022 Jun 16;34:73-84. doi: 10.1016/j.jot.2022.04.004. eCollection 2022 May. J Orthop Translat. 2022. PMID: 35782964 Free PMC article.
-
Incidence of donor site morbidity following harvesting from iliac crest or RIA graft.Injury. 2014 Dec;45 Suppl 6:S116-20. doi: 10.1016/j.injury.2014.10.034. Epub 2014 Oct 27. Injury. 2014. PMID: 25457330 Review.
-
The Reamer-Irrigator-Aspirator System: A Review.Surg Technol Int. 2016 Oct 26;29:287-294. Surg Technol Int. 2016. PMID: 27728952 Review.
Cited by
-
Assessing Cardiopulmonary Safety of Intramedullary Bone Graft Harvesting: A Comparative Study of the RIA 2 System and the ARA Concept.J Orthop Res. 2025 May;43(5):984-993. doi: 10.1002/jor.26059. Epub 2025 Feb 25. J Orthop Res. 2025. PMID: 40001236 Free PMC article.
-
Lost in translation: the lack of agreement between surgeons and scientists regarding biomaterials research and innovation for treating bone defects.BMC Med. 2024 Nov 6;22(1):517. doi: 10.1186/s12916-024-03734-z. BMC Med. 2024. PMID: 39506708 Free PMC article.
-
An injectable calcium sulfate-monetite biphasic cement for the treatment of critical-sized calvarial defects.J Mater Sci Mater Med. 2025 Jul 29;36(1):61. doi: 10.1007/s10856-025-06911-5. J Mater Sci Mater Med. 2025. PMID: 40728601 Free PMC article.
-
Understanding pathophysiology and injury mechanisms is the foundation for invention/innovation and clinical translation in orthopaedics.J Orthop Translat. 2024 Jul 12;47:A1-A2. doi: 10.1016/j.jot.2024.07.003. eCollection 2024 Jul. J Orthop Translat. 2024. PMID: 39161656 Free PMC article. No abstract available.
References
-
- Jia Z., Xu X., Zhu D., Zheng Y. Design, printing, and engineering of regenerative biomaterials for personalized bone healthcare. Prog Mater Sci. 2023;134
-
- Laubach M., Herath B., Bock N., Suresh S., Saifzadeh S., Dargaville B.L., et al. In vivo characterization of 3D-printed polycaprolactone-hydroxyapatite scaffolds with Voronoi design to advance the concept of scaffold-guided bone regeneration. Front Bioeng Biotechnol. 2023;11 [English] - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

