Advancing neoadjuvant therapies in resectable non-small cell lung cancer: implications for novel treatment strategies and biomarker discovery
- PMID: 38957347
- PMCID: PMC11217184
- DOI: 10.3389/pore.2024.1611817
Advancing neoadjuvant therapies in resectable non-small cell lung cancer: implications for novel treatment strategies and biomarker discovery
Abstract
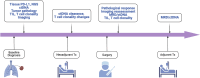
The delivery of neoadjuvant and perioperative therapies for non-small cell lung cancer has been radically altered by significant advances and by the incorporation of targeted therapies as well as immune checkpoint inhibitors alone or alongside conventional chemotherapy. This evolution has been particularly notable in the incorporation of immunotherapy and targeted therapy into the treatment of resectable NSCLC, where recent FDA approvals of drugs such as nivolumab and pembrolizumab, in combination with platinum doublet chemotherapy, have led to considerable improvements in pathological complete response rates and the potential for enhanced long-term survival outcomes. This review emphasizes the growing importance of biomarkers in optimizing treatment selection and explores the impact of emerging studies that challenge existing treatment paradigms and investigate novel therapeutic combinations poised to redefine standard of care practices. Furthermore, the discussion extends to the unmet needs within perioperative treatment assessment and prognostication, highlighting the prospective value of biomarkers in evaluating treatment responses and prognosis.
Keywords: NSCLC; adjuvant; lung cancer; neoadjuvant; perioperative.
Copyright © 2024 Jeon, Gor, D’Aiello, Stiles, Illei and Halmos.
Conflict of interest statement
BH—Receives clinical research funding from Boehringer Ingelheim, Astra Zeneca, Merck, BMS, Advaxis, Amgen, AbbVie, Daiichi, Pfizer, GSK, Beigene, Janssen, Black Diamond Therapeutics, Forward Pharma, Numab, Arrivent. Receives Honoraria from Astra Zeneca, Boehringer Ingelheim, Apollomics, Janssen, Takeda, Merck, BMS, Genentech, Pfizer, Eli-Lilly, Arcus, Merus, Daiichi, Precede. BS—Provides consulting and serves as advisory boards for Medtronic, AstraZeneca, Roche-Genentech, Pfizer, Arcus Biosciences, Bristol Myers Squib, Merck, Regeneron, Galvanize Therapeutics. Receives research funding from BMS Foundation and his wife owns salary/stock for SIGA Technologies. PI—Serves as advisory board for Agilent, AstraZeneca, Sanofi, AbbVie, Genentech, Merus, and speaks for Eli Lilly. Receives research funding from Bristol Myers-Squib. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Vansteenkiste JF, Cho BC, Vanakesa T, De Pas T, Zielinski M, Kim MS, et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2016) 17(6):822–35. 10.1016/S1470-2045(16)00099-1 - DOI - PubMed
-
- Felip E, Rosell R, Maestre JA, Rodríguez-Paniagua JM, Morán T, Astudillo J, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non–small-cell lung cancer. J Clin Oncol (2010) 28(19):3138–45. 10.1200/JCO.2009.27.6204 - DOI - PubMed
-
- Lim E, Harris G, Patel A, Adachi I, Edmonds L, Song F. Preoperative versus postoperative chemotherapy in patients with resectable non-small cell lung cancer: systematic review and indirect comparison meta-analysis of randomized trials. J Thorac Oncol (2009) 4(11):1380–8. 10.1097/JTO.0b013e3181b9ecca - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

