Tuberculosis and Immune Reconstitution Inflammatory Syndrome in Patients With Inflammatory Bowel Disease and Anti-TNFα Treatment: Insights From a French Multicenter Study and Systematic Literature Review With Emphasis on Paradoxical Anti-TNFα Resumption
- PMID: 38957691
- PMCID: PMC11218776
- DOI: 10.1093/ofid/ofae327
Tuberculosis and Immune Reconstitution Inflammatory Syndrome in Patients With Inflammatory Bowel Disease and Anti-TNFα Treatment: Insights From a French Multicenter Study and Systematic Literature Review With Emphasis on Paradoxical Anti-TNFα Resumption
Erratum in
-
Correction to: Tuberculosis and Immune Reconstitution Inflammatory Syndrome in Patients With Inflammatory Bowel Disease and Anti-TNFα Treatment: Insights From a French Multicenter Study and Systematic Literature Review With Emphasis on Paradoxical Anti-TNFα Resumption.Open Forum Infect Dis. 2025 Apr 8;12(4):ofaf208. doi: 10.1093/ofid/ofaf208. eCollection 2025 Apr. Open Forum Infect Dis. 2025. PMID: 40201724 Free PMC article.
Abstract
Background: The advent of anti-tumor necrosis factor α (anti-TNFα) has revolutionized the treatment of inflammatory bowel disease (IBD). However, susceptibility to active tuberculosis (TB) is associated with this therapy and requires its discontinuation. The risk of immune reconstitution inflammatory syndrome (IRIS) in this population is poorly understood, as is the safety of resuming anti-TNFα.
Methods: This French retrospective study (2010-2022) included all TB cases in patients with IBD who were treated with anti-TNFα in 6 participating centers. A systematic literature review was performed on TB-IRIS and anti-TNFα exposure.
Results: Thirty-six patients were included (median age, 35 years; IQR, 27-48). TB was disseminated in 86% and miliary in 53%. IRIS occurred in 47% after a median 45 days (IQR, 18-80). Most patients with TB-IRIS (93%) had disseminated TB. Miliary TB was associated with IRIS risk in univariate analysis (odds ratio, 7.33; 95% CI, 1.60-42.82; P = .015). Anti-TB treatment was longer in this population (median [IQR], 9 [9-12] vs 6 [6-9] months; P = .049). Anti-TNFα was resumed in 66% after a median 4 months (IQR, 3-10) for IBD activity (76%) or IRIS treatment (24%), with only 1 case of TB relapse. Fifty-two cases of TB-IRIS in patients treated with anti-TNFα were reported in the literature, complicating disseminating TB (85%) after a median 42 days (IQR, 21-90), with 70% requiring anti-inflammatory treatment. Forty cases of TB-IRIS or paradoxical reaction treated with anti-TNFα were also reported. IRIS was neurologic in 64%. Outcome was mostly favorable (93% recovery).
Conclusions: TB with anti-TNFα treatment is often complicated by IRIS of varying severity. Restarting anti-TNFα is a safe and effective strategy.
Keywords: TNF-α antagonists; immune reconstitution inflammatory syndrome; infliximab; paradoxical reaction; tuberculosis.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts.
Figures
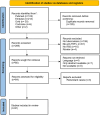

References
-
- Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn's disease: medical treatment. J Crohns Colitis 2020; 14:4–22. - PubMed
-
- Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha–neutralizing agent. N Engl J Med 2001; 345:1098–104. - PubMed
-
- Wallis RS. Mathematical modeling of the cause of tuberculosis during tumor necrosis factor blockade. Arthritis Rheum 2008; 58:947–52. - PubMed
-
- Tubach F, Salmon D, Ravaud P, et al. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: the three-year prospective French Research Axed on Tolerance of Biotherapies registry. Arthritis Rheum 2009; 60:1884–94. - PMC - PubMed
-
- Minozzi S, Bonovas S, Lytras T, et al. Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin Drug Saf 2016; 15(suppl 1):11–34. - PubMed
LinkOut - more resources
Full Text Sources

