Impulse Control Disorders in Southern Iraqi Patients Medicated With Cabergoline for Prolactinoma
- PMID: 38957818
- PMCID: PMC11218533
- DOI: 10.7759/cureus.58516
Impulse Control Disorders in Southern Iraqi Patients Medicated With Cabergoline for Prolactinoma
Abstract
Background: Among the patient population in Basrah, Iraq, prolactinoma is the most commonly found pituitary tumor. Impulse control disorders (ICDs) were reportedly associated with these patients being treated with cabergoline. This study aimed to assess the prevalence of ICDs in cabergoline-treated prolactinoma patients versus healthy, matched controls.
Methods: This cross-sectional case-control study was conducted at the Faiha Specialized Diabetes, Endocrine and Metabolism Center (FDEMC) in Basrah, southern Iraq, from January 2023 to May 2023. It included 30 cabergoline-treated prolactinoma patients and 30 healthy, matched controls. The questionnaire for ICDs in Parkinson's disease was used as a screening tool. Following this, positively screened patients were evaluated using validated criteria accordingly to diagnose impulse control disorders.
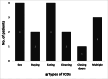
Results: The ICDs were diagnosed in nine (30%) cabergoline-treated prolactinoma patients versus two (6.7%) in control (p = 0.02). The most frequent ICD types were hypersexuality and binge eating, while no patient reported pathological gambling. Three patients reported multiple types of ICDs. The patients' sociodemographic characteristics, prolactinoma duration and size, and cabergoline dose did not correlate significantly with ICD diagnosis.
Conclusions: Treatment with cabergoline is associated with the development of ICDs. Therefore, clinicians should be aware of this disabling side effect to ensure its early detection and treatment.
Keywords: cabergoline; compulsive eating; hypersexual disorder; impulse control disorders; prolactinoma.
Copyright © 2024, Mohammad et al.
Conflict of interest statement
Human subjects: Consent was obtained or waived by all participants in this study. Ethics Committee of Faiha Specialized Diabetes, Endocrine and Metabolism Center (FDEMC) issued approval 12/23/23. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Advances in the treatment of prolactinomas. Gillam MP, Molitch ME, Lombardi G, Colao A. Endocr Rev. 2006;27:485–534. - PubMed
-
- The epidemiology, diagnosis and treatment of Prolactinomas: the old and the new. Chanson P, Maiter D. Best Pract Res Clin Endocrinol Metab. 2019;33:101290. - PubMed
-
- Prolactinomas. Wildemberg LE, Fialho C, Gadelha MR. Presse Med. 2021;50:104080. - PubMed
-
- Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Casanueva FF, Molitch ME, Schlechte JA, et al. Clin Endocrinol (Oxf) 2006;65:265–273. - PubMed
LinkOut - more resources
Full Text Sources