Valence photoelectron imaging of molecular oxybenzone
- PMID: 38957915
- PMCID: PMC11253247
- DOI: 10.1039/d3cp06224d
Valence photoelectron imaging of molecular oxybenzone
Abstract
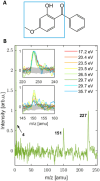
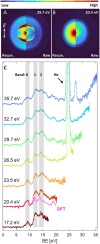
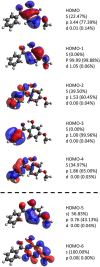
An oxybenzone molecule in the gas phase was characterized by mass spectrometry and angle-resolved photoelectron spectroscopy, using both single and multiphoton ionization schemes. A tabletop high harmonic generation source with a monochromator was used for single-photon ionization of oxybenzone with photon energies of up to 35.7 eV. From this, vertical ionization and appearance energies, as well as energy-dependent anisotropy parameters were retrieved and compared with the results from DFT calculations. For two-photon ionization using 4.7 eV light, we found a higher appearance energy than in the extreme ultraviolet (EUV) case, highlighting the possible influence of an intermediate state on the photoionization process. We found no differences in the mass spectra when ionizing oxybenzone by single-photons between 17.2 and 35.7 eV. However, for the multiphoton ionization, the fragmentation process was found to be sensitive to the photoionization order and laser intensity. The "softest" method was found to be two-photon ionization using 4.7 eV light, which led to no measurable fragmentation up to an intensity of 5 × 1012 W cm-2.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Downs C. A. Kramarsky-Winter E. Segal R. Fauth J. Knutson S. Bronstein O. Ciner F. R. Jeger R. Lichtenfeld Y. Woodley C. M. Pennington P. Cadenas K. Kushmaro A. Loya Y. Toxicopathological Effects of the Sunscreen UV Filter, Oxybenzone (Benzophenone-3), on Coral Planulae and Cultured Primary Cells and Its Environmental Contamination in Hawaii and the U.S. Virgin Islands. Arch. Environ. Contam. Toxicol. 2016;70:265–288. doi: 10.1007/s00244-015-0227-7. - DOI - PubMed
-
- Baker L. A. Horbury M. D. Greenough S. E. Coulter P. M. Karsili T. N. V. Roberts G. M. Orr-Ewing A. J. Ashfold M. N. R. Stavros V. G. Probing the Ultrafast Energy Dissipation Mechanism of the Sunscreen Oxybenzone after UVA Irradiation. J. Phys. Chem. Lett. 2015;6:1363–1368. doi: 10.1021/acs.jpclett.5b00417. - DOI - PubMed
LinkOut - more resources
Full Text Sources

