Heterogeneous treatment effects of sodium-glucose cotransporter 2 inhibitors on risk of dementia in people with type 2 diabetes: A population-based cohort study
- PMID: 38958394
- PMCID: PMC11350016
- DOI: 10.1002/alz.14048
Heterogeneous treatment effects of sodium-glucose cotransporter 2 inhibitors on risk of dementia in people with type 2 diabetes: A population-based cohort study
Abstract
Introduction: Sodium-glucose cotransporter 2 (SGLT2) inhibitors exhibit potential benefits in reducing dementia risk, yet the optimal beneficiary subgroups remain uncertain.
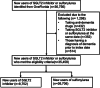
Methods: Individuals with type 2 diabetes (T2D) initiating either SGLT2 inhibitor or sulfonylurea were identified from OneFlorida+ Clinical Research Network (2016-2022). A doubly robust learning was deployed to estimate risk difference (RD) and 95% confidence interval (CI) of all-cause dementia.
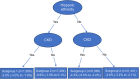
Results: Among 35,458 individuals with T2D, 1.8% in the SGLT2 inhibitor group and 4.7% in the sulfonylurea group developed all-cause dementia over a 3.2-year follow-up, yielding a lower risk for SGLT2 inhibitors (RD, -2.5%; 95% CI, -3.0% to -2.1%). Hispanic ethnicity and chronic kidney disease were identified as the two important variables to define four subgroups in which RD ranged from -4.3% (-5.5 to -3.2) to -0.9% (-1.9 to 0.2).
Discussion: Compared to sulfonylureas, SGLT2 inhibitors were associated with a reduced risk of all-cause dementia, but the association varied among different subgroups.
Highlights: New users of sodium-glucose cotransporter 2 (SGLT2) inhibitors were significantly associated with a lower risk of all-cause dementia as compared to those of sulfonylureas. The association varied among different subgroups defined by Hispanic ethnicity and chronic kidney disease. A significantly lower risk of Alzheimer's disease and vascular dementia was observed among new users of SGLT2 inhibitors compared to those of sulfonylureas.
Keywords: Alzheimer's disease; SGLT2 inhibitors; dementia; heterogeneous treatment effects; sulfonylureas; type 2 diabetes.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
H.T. is supported by the Pharmaceutical Research and Manufacturers of America (PhRMA) Foundation Predoctoral Fellowship and American Foundation for Pharmaceutical Education (AFPE) Predoctoral Fellowship. C.E.S. reports that she is funded by National Institute on Aging (NIA; K01AG071849) and she is also the Chair of the ISTAART Professional Interest Area to Elevate Early Career Researchers and Co‐Chair of the ISTAART Sex and Gender Interest Group, Diversity and Disparities Professional Interest Area. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. S.T.D. received royalty from UpToDate and consulting fee from Brainstorm Cell Therapeutics; he also participated on the Acumen Pharmaceuticals Medical Advisory Board, Biogen Data Safety Monitoring Board (DSMB), Cognition Therapeutics Medical Advisory Board, Prevail Pharmaceuticals DSMB, and Vaccinex Medical Advisory Board; they are outside of the current work. J.B. is supported by NIA (1R01AG076234) and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R01DK133465). J.G. is supported by NIDDK (R01DK133465). She received consulting fees from Pfizer Inc. (outside the current work). All other authors declare no conflict of interest. Author disclosures are available in the supporting information.
Figures



References
-
- Barker WW, Luis CA, Kashuba A, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16:203‐212. - PubMed
-
- 2023 Alzheimer's disease facts and figures. Alzheimers Dement. 2023;19:1598‐1695. - PubMed
-
- Centers for Disease Control and Prevention . Alzheimer's Disease and Healthy Aging. 2019. Accessed May 13, 2022. Available at https://www.cdc.gov/aging/dementia/index.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

