Efficacy of T-cell assays for the diagnosis of primary defects in cytotoxic lymphocyte exocytosis
- PMID: 38958468
- PMCID: PMC11375501
- DOI: 10.1182/blood.2024024499
Efficacy of T-cell assays for the diagnosis of primary defects in cytotoxic lymphocyte exocytosis
Abstract
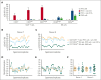
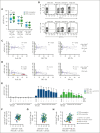
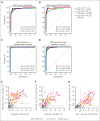
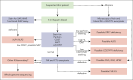
Primary hemophagocytic lymphohistiocytosis (HLH) is a life-threatening disorder associated with autosomal recessive variants in genes required for perforin-mediated lymphocyte cytotoxicity. A rapid diagnosis is crucial for successful treatment. Although defective cytotoxic T lymphocyte (CTL) function causes pathogenesis, quantification of natural killer (NK)-cell exocytosis triggered by K562 target cells currently represents a standard diagnostic procedure for primary HLH. We have prospectively evaluated different lymphocyte exocytosis assays in 213 patients referred for evaluation for suspected HLH and related hyperinflammatory syndromes. A total of 138 patients received a molecular diagnosis consistent with primary HLH. Assessment of Fc receptor-triggered NK-cell and T-cell receptor (TCR)-triggered CTL exocytosis displayed higher sensitivity and improved specificity for the diagnosis of primary HLH than routine K562 cell-based assays, with these assays combined providing a sensitivity of 100% and specificity of 98.3%. By comparison, NK-cell exocytosis after K562 target cell stimulation displayed a higher interindividual variability, in part explained by differences in NK-cell differentiation or large functional reductions after shipment. We thus recommend combined analysis of TCR-triggered CTL and Fc receptor-triggered NK-cell exocytosis for the diagnosis of patients with suspected familial HLH or atypical manifestations of congenital defects in lymphocyte exocytosis.
Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of all pHLH collaborators appears in “Appendix.”
Figures


Comment in
-
Redirect your attention: new CTL assay for HLH.Blood. 2024 Aug 22;144(8):802-804. doi: 10.1182/blood.2024025526. Blood. 2024. PMID: 39172442 No abstract available.
References
-
- Henter JI, Horne A, Arico M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

