Nanoscale flow cytometry-based quantification of blood-based extracellular vesicle biomarkers distinguishes MCI and Alzheimer's disease
- PMID: 38958575
- PMCID: PMC11497682
- DOI: 10.1002/alz.14087
Nanoscale flow cytometry-based quantification of blood-based extracellular vesicle biomarkers distinguishes MCI and Alzheimer's disease
Abstract
Introduction: Accurate testing for Alzheimer's disease (AD) represents a crucial step for therapeutic advancement. Currently, tests are expensive and require invasive sampling or radiation exposure.
Methods: We developed a nanoscale flow cytometry (nFC)-based assay of extracellular vesicles (EVs) to screen biomarkers in plasma from mild cognitive impairment (MCI), AD, or controls.
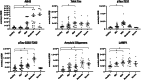
Results: Circulating amyloid beta (Aβ), tau, phosphorylated tau (p-tau)181, p-tau231, p-tau217, p-tauS235, ubiquitin, and lysosomal-associated membrane protein 1-positive EVs distinguished AD samples. p-tau181, p-tau217, p-tauS235, and ubiquitin-positive EVs distinguished MCI samples. The most sensitive marker for AD distinction was p-tau231, with an area under the receiver operating characteristic curve (AUC) of 0.96 (sensitivity 0.95/specificity 1.0) improving to an AUC of 0.989 when combined with p-tauS235.
Discussion: This nFC-based assay accurately distinguishes MCI and AD plasma without EV isolation, offering a rapid approach requiring minute sample volumes. Incorporating nFC-based measurements in larger populations and comparison to "gold standard" biomarkers is an exciting next step for developing AD diagnostic tools.
Highlights: Extracellular vesicles represent promising biomarkers of Alzheimer's disease (AD) that can be measured in the peripheral circulation. This study demonstrates the utility of nanoscale flow cytometry for the measurement of circulating extracellular vesicles (EVs) in AD blood samples. Multiple markers including amyloid beta, tau, phosphorylated tau (p-tau)181, p-tau231, p-tau217, and p-tauS235 accurately distinguished AD samples from healthy controls. Future studies should expand blood and cerebrospinal fluid-based EV biomarker development using nanoflow cytometry approaches.
Keywords: Alzheimer's disease; biomarker; dementia; extracellular vesicle; mild cognitive impairment; nanoflow cytometry.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflicts of interest. Author disclosures are available in the supporting information.
Figures





References
-
- 2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17:327‐406. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

