Eteplirsen Treatment for Duchenne Muscular Dystrophy: A Qualitative Patient Experience Study
- PMID: 38958840
- PMCID: PMC11263411
- DOI: 10.1007/s12325-024-02915-9
Eteplirsen Treatment for Duchenne Muscular Dystrophy: A Qualitative Patient Experience Study
Abstract
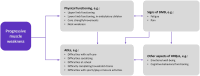
Introduction: Duchenne muscular dystrophy (DMD) is characterized by rapid functional decline. Current available treatment options aim to delay disease progression or stabilize physical function. To aid in healthcare providers' understanding of the symptoms of disease that impact patients' experience, this study explored children's physical functioning, activities of daily living (ADLs), and health-related quality of life (HRQoL) after receiving eteplirsen, a weekly infusion indicated for individuals with DMD with exon 51 skip-amenable mutations.
Methods: Fifteen caregivers of male individuals with DMD participated in a 60-min, semi-structured interview. Open-ended questioning explored changes in the children's condition or maintenance in abilities since eteplirsen initiation.
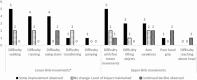
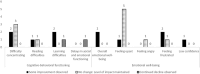
Results: Children with DMD (age 7-15 years [mean 10.9]; steroid treatment at interview, n = 8; time since eteplirsen initiation 3-24 months [mean 14.9]) were described by caregivers as ambulatory (n = 9) and non-ambulatory (n = 6). Caregivers of ambulatory children reported improvements or maintenance of walking ability (n = 7/9), running (n = 6/9), and using stairs (n = 4/9). Continued decline in using stairs was reported by two caregivers. In upper-limb functioning, improvements or maintenances in fine-motor movements were reported by nearly half of all caregivers (n = 7/15), with one caregiver noting a continued decline. Subsequent improvements or maintenances in ADLs were described. Improvements or maintenances in fatigue (n = 9/15), muscle weakness (n = 7/15), and pain (n = 6/15) were reported, although some caregivers described a continued decline (n = 3/15 fatigue, n = 1/15 muscle weakness, n = 2/15 pain). Importantly, most caregivers who reported maintenances in ability perceived this as a positive outcome (n = 6/9).
Conclusion: This exploratory study indicated that most caregivers perceived improvements or maintenances in aspects of their child's physical functioning, ADLs, and HRQoL since eteplirsen initiation, which they perceived to be a positive outcome.
Keywords: Duchenne muscular dystrophy; Eteplirsen; Health-related quality of life; Qualitative.
Plain language summary
Duchenne muscular dystrophy (DMD) is a rare disease characterized by progressive muscle weakness. Early on, this weakness presents as difficulty walking, but eventually children lose the ability to walk, develop spinal curvature, and experience problems with the heart and lung muscles. People with DMD are missing a key protein in their bodies called dystrophin. Eteplirsen is a weekly, intravenous treatment approved to treat people with a specific DMD genetic misspelling. The goal of the treatment is to slow down the disease and delay the time to losing ability to walk or needing help breathing. Fifteen caregivers of children living with DMD participated in a 60-min telephone interview. Caregivers were asked questions about the child’s DMD symptoms and how those symptoms impact the child’s daily life. Caregivers discussed their child’s experience while receiving eteplirsen treatment and changes since the start of treatment. Caregivers described their child’s muscle weakness and how this has affected their movements (e.g., using stairs, running or walking). Since starting eteplirsen treatment, all caregivers reported some improvement or maintenance in parts of their child’s physical functioning, activities of daily living (e.g., sports/leisure, getting dressed and self-care), and symptoms (e.g., muscle weakness, pain and fatigue), even though some decline was also reported (e.g., physical functioning, getting dressed, self-care, muscle weakness, pain and fatigue). The results provide insights into physical functioning and quality of life of children with DMD who are receiving eteplirsen. However, more research is needed to fully understand the impact of eteplirsen on these experiences.
© 2024. The Author(s).
Conflict of interest statement
Joel Iff, Ihor Sehinovych, and Carolyn McNeill are employees of Sarepta Therapeutics, Inc., and may hold stock/options. Chloe Carmichael, Stephanie McKee, and Helen Kitchen are employees and stockholders of Clarivate, a healthcare research company that provides consultancy to pharmaceutical companies, including Sarepta Therapeutics, Inc. Carolina Tesi-Rocha reports consulting fees (Avexis, Biogen, Sarepta Therapeutics, Inc., Novartis, NS Pharma) and is a site investigator for clinical trials: Avexis, Biogen, Cytokinetics, Genzyme, Pfizer, PTC Therapeutics, Roche, Sarepta Therapeutics, Inc., Scholar Rock, and Janssen. Erik Henricson has received consulting fees from Sarepta Therapeutics, Inc., Santhera Pharmaceuticals, Pfizer, Eprirum Bio, Capricor, Catabasis, Mallinkrodt, Bristol-Myers Squibb, PTC Therapeutics, PepGen, and GSK and has received speaker honoraria from Parent Project Muscular Dystrophy, Muscular Dystrophy Association, and ENMC. Francesco Muntoni has received consultant fees and speaker honoraria from Sarepta Therapeutics, Inc. He is a member of the Pfizer SAB and, relevant for DMD, has received consultancies from Dyne Therapeutics, Roche, and PTC Therapeutics.
Figures