Morphological and Molecular Characteristics of Perineuronal Nets in the Human Prefrontal Cortex-A Possible Link to Microcircuitry Specialization
- PMID: 38958887
- PMCID: PMC11711633
- DOI: 10.1007/s12035-024-04306-1
Morphological and Molecular Characteristics of Perineuronal Nets in the Human Prefrontal Cortex-A Possible Link to Microcircuitry Specialization
Abstract
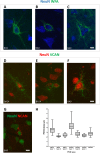
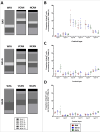
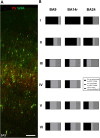
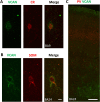
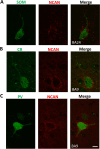
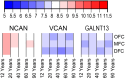
Perineuronal nets (PNNs) are a type of extracellular matrix (ECM) that play a significant role in synaptic activity and plasticity of interneurons in health and disease. We researched PNNs' regional and laminar representation and molecular composition using immunohistochemistry and transcriptome analysis of Brodmann areas (BA) 9, 14r, and 24 in 25 human postmortem brains aged 13-82 years. The numbers of VCAN- and NCAN-expressing PNNs, relative to the total number of neurons, were highest in cortical layers I and VI while WFA-binding (WFA+) PNNs were most abundant in layers III-V. The ECM glycosylation pattern was the most pronounced regional difference, shown by a significantly lower proportion of WFA+ PNNs in BA24 (3.27 ± 0.69%) compared to BA9 (6.32 ± 1.73%; P = 0.0449) and BA14 (5.64 ± 0.71%; P = 0.0278). The transcriptome of late developmental and mature stages revealed a relatively stable expression of PNN-related transcripts (log2-transformed expression values: 6.5-8.5 for VCAN and 8.0-9.5 for NCAN). Finally, we propose a classification of PNNs that envelop GABAergic neurons in the human cortex. The significant differences in PNNs' morphology, distribution, and molecular composition strongly suggest an involvement of PNNs in specifying distinct microcircuits in particular cortical regions and layers.
Keywords: Extracellular matrix; GABA; Interneurons; Neurocan; Versican.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics Approval: This study was approved by the Ethics Committee of Zagreb University School of Medicine (380-59-10106-14-55/152). Consent to Participate: Not applicable. Consent for Publication: Not applicable. Competing Interests: The authors declare no competing interests.
Figures



References
-
- Benarroch EE (2015) Extracellular matrix in the CNS: dynamic structure and clinical correlations. Neurology 85:1417–1427. 10.1212/WNL.0000000000002044 - PubMed
-
- Jovanov Milošević N, Judaš M, Aronica E et al (2014) Neural ECM in laminar organization and connectivity development in healthy and diseased human brain. Prog Brain Res 214:159–178. 10.1016/B978-0-444-63486-3.00007-4 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

