Interactive Media-Based Approach for an Exception From Informed Consent Trial Involving Patients With Trauma
- PMID: 38959020
- PMCID: PMC11223059
- DOI: 10.1001/jamasurg.2024.2147
Interactive Media-Based Approach for an Exception From Informed Consent Trial Involving Patients With Trauma
Abstract
Importance: Exception From Informed Consent (EFIC) research requires community consultation (CC) and public disclosure (PD). Traditional methods of conducting CC and PD are slow, expensive, and labor intensive.
Objective: To describe the feasibility and reach of a novel interactive, media-based approach to CC and PD and to identify the similarities and differences between trial sites in website views, survey responses, online community forum attendance, and opt-out requests.
Design, setting, and participants: This survey study analyzed the CC and PD campaigns conducted for the TAP trial (Evaluation of BE1116 in Patients With Traumatic Injury and Acute Major Bleeding to Improve Survival), an EFIC trial of the early administration of prothrombin complex concentrate in patients with trauma. The CC and PD campaigns consisted of social media advertisements, linked websites, community surveys, and online community forums. These activities were coordinated from a central site and approved by a central institutional review board. This study focused on the first 52 of 91 TAP trial sites (level I trauma centers) in the US to have completed their CC and PD campaigns. Community members in the catchment areas of the participating trauma centers were targeted. Data analysis was conducted between October 2023 and February 2024.
Exposure: Social media advertisements, surveys, and online community meetings conducted as part of the CC and PD campaign for the TAP trial.
Main outcomes and measures: Social media campaign reach and engagement, web page views, survey results, online community forum attendance, and opt-out requests.
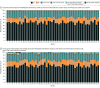
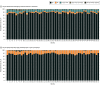
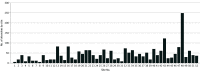
Results: Fifty-two trial sites were approved for participant enrollment. Social media advertisements were displayed 92 million times, reaching 11.8 million individuals. The median (IQR) number of people reached in each location was 210 317 (172 068-276 968). Site-specific websites were viewed 144 197 times (median [IQR] viewings per site, 2984 [1267-4038]). A total of 17 206 fully or partly completed surveys were received, and survey respondents had a median (IQR) age of 40.1 (15-65) years and included 10 444 females (60.7%). Overall, 60.6% survey respondents said they would want to be entered into the trial even if they could not give consent, 87.7% agreed that emergency care research was necessary, and 88.0% agreed that the TAP trial should be conducted in their community. Online community forums were attended by a median (IQR) number of 38 (20-63) people. Four opt-out requests were received.
Conclusions and relevance: The interactive media-based approach to CC and PD for the ongoing TAP trial showed the feasibility and benefits of executing an efficient, coordinated, centrally run series of locally branded and geographically targeted CC and PD campaigns for a large EFIC study.
Conflict of interest statement
Figures

Comment on
-
Exception From Informed Consent-A Model for Donor Intervention Research.JAMA Surg. 2024 Sep 1;159(9):1058-1059. doi: 10.1001/jamasurg.2024.2113. JAMA Surg. 2024. PMID: 38959004 No abstract available.
References
-
- Federal Register. 21 CFR 50.24 Exception from informed consent requirements for emergency research. Fed Regist. 1996;61(192):51498-51531. - PubMed
-
- Food and Drug Administration . Exception from informed consent requirements for emergency research (21 CFR 50.24). Accessed January 25, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

