From hit to vial: Precision discovery and development of an imidazopyrimidine TLR7/8 agonist adjuvant formulation
- PMID: 38959314
- PMCID: PMC11221515
- DOI: 10.1126/sciadv.adg3747
From hit to vial: Precision discovery and development of an imidazopyrimidine TLR7/8 agonist adjuvant formulation
Abstract
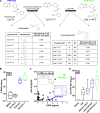
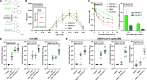
Vaccination can help prevent infection and can also be used to treat cancer, allergy, and potentially even drug overdose. Adjuvants enhance vaccine responses, but currently, the path to their advancement and development is incremental. We used a phenotypic small-molecule screen using THP-1 cells to identify nuclear factor-κB (NF-κB)-activating molecules followed by counterscreening lead target libraries with a quantitative tumor necrosis factor immunoassay using primary human peripheral blood mononuclear cells. Screening on primary cells identified an imidazopyrimidine, dubbed PVP-037. Moreover, while PVP-037 did not overtly activate THP-1 cells, it demonstrated broad innate immune activation, including NF-κB and cytokine induction from primary human leukocytes in vitro as well as enhancement of influenza and SARS-CoV-2 antigen-specific humoral responses in mice. Several de novo synthesis structural enhancements iteratively improved PVP-037's in vitro efficacy, potency, species-specific activity, and in vivo adjuvanticity. Overall, we identified imidazopyrimidine Toll-like receptor-7/8 adjuvants that act in synergy with oil-in-water emulsion to enhance immune responses.
Figures

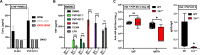

References
-
- Finn O. J., The dawn of vaccines for cancer prevention. Nat. Rev. Immunol. 18, 183–194 (2018). - PubMed
-
- Raleigh M. D., Laudenbach M., Baruffaldi F., Peterson S. J., Roslawski M. J., Birnbaum A. K., Carroll F. I., Runyon S. P., Winston S., Pentel P. R., Pravetoni M., Opioid dose- and route-dependent efficacy of oxycodone and heroin vaccines in rats. J. Pharmacol. Exp. Ther. 365, 346–353 (2018). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

