Tissue-based T cell activation and viral RNA persist for up to 2 years after SARS-CoV-2 infection
- PMID: 38959327
- PMCID: PMC11337933
- DOI: 10.1126/scitranslmed.adk3295
Tissue-based T cell activation and viral RNA persist for up to 2 years after SARS-CoV-2 infection
Abstract
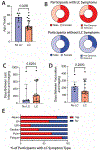
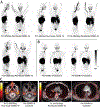
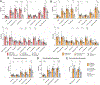
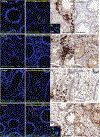
The mechanisms of postacute medical conditions and unexplained symptoms after SARS-CoV-2 infection [Long Covid (LC)] are incompletely understood. There is growing evidence that viral persistence, immune dysregulation, and T cell dysfunction may play major roles. We performed whole-body positron emission tomography imaging in a well-characterized cohort of 24 participants at time points ranging from 27 to 910 days after acute SARS-CoV-2 infection using the radiopharmaceutical agent [18F]F-AraG, a selective tracer that allows for anatomical quantitation of activated T lymphocytes. Tracer uptake in the postacute COVID-19 group, which included those with and without continuing symptoms, was higher compared with prepandemic controls in many regions, including the brain stem, spinal cord, bone marrow, nasopharyngeal and hilar lymphoid tissue, cardiopulmonary tissues, and gut wall. T cell activation in the spinal cord and gut wall was associated with the presence of LC symptoms. In addition, tracer uptake in lung tissue was higher in those with persistent pulmonary symptoms specifically. Increased T cell activation in these tissues was also observed in many individuals without LC. Given the high [18F]F-AraG uptake detected in the gut, we obtained colorectal tissue for in situ hybridization of SARS-CoV-2 RNA and immunohistochemical studies in a subset of five participants with LC symptoms. We identified intracellular SARS-CoV-2 single-stranded spike protein-encoding RNA in rectosigmoid lamina propria tissue in all five participants and double-stranded spike protein-encoding RNA in three participants up to 676 days after initial COVID-19, suggesting that tissue viral persistence could be associated with long-term immunologic perturbations.
Conflict of interest statement
Conflicts of Interest
MJP reports consulting fees for Gilead Sciences and AstraZeneca and research support from Aerium Therapeutics, outside the submitted work. SGD reports consulting for Enanta Pharmaceuticals and Pfizer and research support from Aerium Therapeutics, outside the submitted work. TJH reports consulting fees for Roche and Regeneron outside the submitted work. JL is employed by CellSight Technologies, holds CellSight stock options, and holds patents relating to [18F]F-AraG (Methods and Materials for Making PET Radiotracers, US20220370648A1; Methods and Materials for Using [18F]F-AraG in Cardiac Imaging, US20230309833A1). AG was employed by CellSight Technologies.
Figures


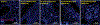
References
-
- Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, Ahluwalia N, Bikdeli B, Dietz D, Der-Nigoghossian C, Liyanage-Don N, Rosner GF, Bernstein EJ, Mohan S, Beckley AA, Seres DS, Choueiri TK, Uriel N, Ausiello JC, Accili D, Freedberg DE, Baldwin M, Schwartz A, Brodie D, Garcia CK, Elkind MSV, Connors JM, Bilezikian JP, Landry DW, Wan EY, Post-acute COVID-19 syndrome. Nat Med 27, 601–615 (2021). - PMC - PubMed
-
- Al-Aly Z, Xie Y, Bowe B, High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594, 259–264 (2021). - PubMed
-
- Centers for Disease Control and Prevention COVID Data Tracker. US Department of Health and Human Services, CDC. (https://covid.cdc.gov/covid-data-tracker).
-
- Robertson MM, Qasmieh SA, Kulkarni SG, Teasdale CA, Jones HE, McNairy M, Borrell LN, Nash D, The Epidemiology of Long Coronavirus Disease in US Adults. Clin Infect Dis 76, 1636–1645 (2023). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

