Anticoagulant Medications: The Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE Consensus Conference
- PMID: 38959355
- PMCID: PMC11216397
- DOI: 10.1097/PCC.0000000000003495
Anticoagulant Medications: The Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE Consensus Conference
Abstract
Objectives: To derive systematic-review informed, modified Delphi consensus regarding the medications used for anticoagulation for pediatric extracorporeal membrane oxygenation (ECMO) for the Pediatric ECMO Anticoagulation CollaborativE (PEACE).
Data sources: A structured literature search was performed using PubMed, EMBASE, and Cochrane Library (CENTRAL) databases from January 1988 to May 2021.
Study selection: Included studies assessed anticoagulation used in pediatric ECMO.
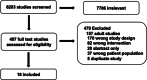
Data extraction: Two authors reviewed all citations independently, with a third reviewer adjudicating any conflicts. Eighteen references were used for data extraction as well as for creation of recommendations. Evidence tables were constructed using a standardized data extraction form.
Data synthesis: Risk of bias was assessed using the Quality in Prognosis Studies tool. The evidence was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation system. Forty-eight experts met over 2 years to develop evidence-informed recommendations and, when evidence was lacking, expert-based consensus statements, or good practice statements for anticoagulation during pediatric ECMO. A web-based modified Delphi process was used to build consensus via the Research and Development/University of California Appropriateness Method. Consensus was based on a modified Delphi process with agreement defined as greater than 80%. Two recommendations, two consensus statements, and one good practice statement were developed, and, in all, agreement greater than 80% was reached.
Conclusions: There is insufficient evidence to formulate optimal anticoagulation therapy during pediatric ECMO. Additional high-quality research is needed to inform evidence-based practice for anticoagulation during pediatric ECMO.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies.
Conflict of interest statement
The Executive Committee (Drs. Alexander, Muszynski, Bembea, Cheifetz, Steiner, and Barbaro) served as arbitrators for conflict of interest management. Dr. Alexander’s institution received funding from Novartis (ProspectiveTrial to Assess the Angiotensin Receptor Blocker Neprilysin Inhibitor LCZ696 Versus Angiotensin-Converting Enzyme Inhibitor for the Medical Treatment of Pediatric HF [PANORAMA-HF]). Dr. Sloan commenced employment with CSL Behring after the consensus process was complete. Dr. Patregnani discloses consultation payments from MNK Pharmaceuticals and Pfizer. Dr. Dalton discloses she is a consultant for Innovative Extracorporeal Membrane Oxygenation Concepts, Hemocue Entegrion, and Medtronic. Dr. Ryerson received an honorarium from the Instrumentation Laboratory for consultation work. Drs. Brandão, Monagle, Ryerson, Alexander, and Dalton disclosed the off-label product use of direct thrombin inhibitors for pediatric ECMO anticoagulation. Dr. Muszynski’s institution received funding from the National Institutes of Health (NIH). Drs. Muszynski and Alexander received support for article research from the NIH. Dr. Alexander’s institution received funding from the National Institute of Child Health and Human Development (R13HD104432), ELSO, and Novartis. Dr. Dalton received funding from Innovative ECMO Concepts, Entegrion, and Hemocue. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures
References
-
- Dalton HJ, Reeder R, Garcia-Filion P, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network: Factors associated with bleeding and thrombosis in children receiving extracorporeal membrane oxygenation. Am J Respir Crit Care Med. 2017; 196:762–771 - PMC - PubMed
-
- O’Halloran CP, Andren KG, Mecklosky J, et al. : Mortality and factors associated with hemorrhage during pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2020; 21:75–81 - PubMed
-
- Muszynski JA, Reeder RW, Hall MW, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN): RBC transfusion practice in pediatric extracorporeal membrane oxygenation support. Crit Care Med. 2018; 46:e552–e559 - PMC - PubMed
-
- Ozment CP, Scott BL, Bembea MM, et al. : Anticoagulation and transfusion management during neonatal and pediatric extracorporeal membrane oxygenation: A survey of medical directors in the United States. Pediatr Crit Care Med. 2021; 22:530–541 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous