A phase Ib randomized multicenter trial of isolated hepatic perfusion in combination with ipilimumab and nivolumab for uveal melanoma metastases (SCANDIUM II trial)
- PMID: 38959698
- PMCID: PMC11269777
- DOI: 10.1016/j.esmoop.2024.103623
A phase Ib randomized multicenter trial of isolated hepatic perfusion in combination with ipilimumab and nivolumab for uveal melanoma metastases (SCANDIUM II trial)
Abstract
Background: Uveal melanoma (UM) is a rare malignancy where 50% of patients develop metastatic disease primarily affecting the liver. Approximately 40% of patients with metastatic UM respond to one-time isolated hepatic perfusion (IHP) with high-dose melphalan. This phase I trial investigates the safety and clinical efficacy of IHP combined with ipilimumab (IPI) and nivolumab (NIVO).
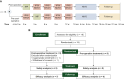
Patients and methods: Immunotherapy-naïve patients were randomized in this phase I trial to receive either IHP followed by IPI 3 mg/kg and NIVO 1 mg/kg (IPI3/NIVO1) for four cycles (post-operative arm), or one cycle of preoperative IPI3/NIVO1, IHP and then three cycles of IPI3/NIVO1 (pre-post-operative arm), followed by maintenance therapy with NIVO 480 mg for 1 year.
Results: Eighteen patients were enrolled and randomized. Three patients did not undergo IHP as planned. In total, 11/18 patients (6 in the post-operative arm and 5 in the pre-post-operative arm) did not complete the planned four cycles of IPI3/NIVO1. Toxicity to IHP was similar in both groups, but the number of immune-related adverse events (AEs) was higher in the pre-post-operative arm. Among assessable patients, overall response rate was 57% in the post-operative arm (4/7) and 22% in the pre-post-operative arm (2/9).
Conclusions: Combination therapy with IHP and IPI3/NIVO1 was associated with severe AEs. The efficacy of this combination is encouraging with high response rates. One cycle of preoperative IPI/NIVO before IHP did not show potential benefits in terms of safety or efficacy.
Keywords: CTLA-4; PD-1; immune checkpoint inhibitor; isolated hepatic perfusion; liver metastases; uveal melanoma.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Diener-West M., Reynolds S.M., Agugliaro D.J., et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005;123(12):1639–1643. - PubMed
-
- Khoja L., Atenafu E.G., Suciu S., et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: an international rare cancers initiative (IRCI) ocular melanoma study. Ann Oncol. 2019;30(8):1370–1380. - PubMed
-
- Piulats J.M., Espinosa E., de la Cruz Merino L., et al. Nivolumab plus ipilimumab for treatment-naïve metastatic uveal melanoma: an open-label, multicenter, phase II trial by the Spanish Multidisciplinary Melanoma Group (GEM-1402) J Clin Oncol. 2021;39(6):586–598. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

