The germline coordinates mitokine signaling
- PMID: 38959891
- PMCID: PMC12261959
- DOI: 10.1016/j.cell.2024.06.010
The germline coordinates mitokine signaling
Abstract
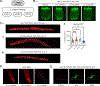
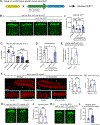
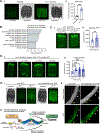
The ability of mitochondria to coordinate stress responses across tissues is critical for health. In C. elegans, neurons experiencing mitochondrial stress elicit an inter-tissue signaling pathway through the release of mitokine signals, such as serotonin or the Wnt ligand EGL-20, which activate the mitochondrial unfolded protein response (UPRMT) in the periphery to promote organismal health and lifespan. We find that germline mitochondria play a surprising role in neuron-to-periphery UPRMT signaling. Specifically, we find that germline mitochondria signal downstream of neuronal mitokines, Wnt and serotonin, and upstream of lipid metabolic pathways in the periphery to regulate UPRMT activation. We also find that the germline tissue itself is essential for UPRMT signaling. We propose that the germline has a central signaling role in coordinating mitochondrial stress responses across tissues, and germline mitochondria play a defining role in this coordination because of their inherent roles in germline integrity and inter-tissue signaling.
Keywords: C. elegans; aging; germline; lipids; mitochondria; mtUPR; proteostasis; stress response.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Update of
-
The germline coordinates mitokine signaling.bioRxiv [Preprint]. 2023 Oct 4:2023.08.21.554217. doi: 10.1101/2023.08.21.554217. bioRxiv. 2023. Update in: Cell. 2024 Aug 22;187(17):4605-4620.e17. doi: 10.1016/j.cell.2024.06.010. PMID: 37873079 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

