miR-107 reverses the multidrug resistance of gastric cancer by targeting the CGA/EGFR/GATA2 positive feedback circuit
- PMID: 38960034
- PMCID: PMC11345541
- DOI: 10.1016/j.jbc.2024.107522
miR-107 reverses the multidrug resistance of gastric cancer by targeting the CGA/EGFR/GATA2 positive feedback circuit
Abstract
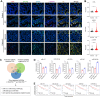
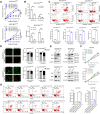
Chemotherapy is still the main therapeutic strategy for gastric cancer (GC). However, most patients eventually acquire multidrug resistance (MDR). Hyperactivation of the EGFR signaling pathway contributes to MDR by promoting cancer cell proliferation and inhibiting apoptosis. We previously identified the secreted protein CGA as a novel ligand of EGFR and revealed a CGA/EGFR/GATA2 positive feedback circuit that confers MDR in GC. Herein, we outline a microRNA-based treatment approach for MDR reversal that targets both CGA and GATA2. We observed increased expression of CGA and GATA2 and increased activation of EGFR in GC samples. Bioinformatic analysis revealed that miR-107 could simultaneously target CGA and GATA2, and the low expression of miR-107 was correlated with poor prognosis in GC patients. The direct interactions between miR-107 and CGA or GATA2 were validated by luciferase reporter assays and Western blot analysis. Overexpression of miR-107 in MDR GC cells increased their susceptibility to chemotherapeutic agents, including fluorouracil, adriamycin, and vincristine, in vitro. Notably, intratumor injection of the miR-107 prodrug enhanced MDR xenograft sensitivity to chemotherapies in vivo. Molecularly, targeting CGA and GATA2 with miR-107 inhibited EGFR downstream signaling, as evidenced by the reduced phosphorylation of ERK and AKT. These results suggest that miR-107 may contribute to the development of a promising therapeutic approach for the treatment of MDR in GC.
Keywords: CGA; EGFR signaling; GATA2; gastric cancer; miRNA; multidrug resistance.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



Similar articles
-
A CGA/EGFR/GATA2 positive feedback circuit confers chemoresistance in gastric cancer.J Clin Invest. 2022 Mar 15;132(6):e154074. doi: 10.1172/JCI154074. J Clin Invest. 2022. PMID: 35289315 Free PMC article.
-
Downregulation of leucine-rich repeats and immunoglobulin-like domains 1 by microRNA-20a modulates gastric cancer multidrug resistance.Cancer Sci. 2018 Apr;109(4):1044-1054. doi: 10.1111/cas.13538. Epub 2018 Mar 23. Cancer Sci. 2018. PMID: 29450946 Free PMC article.
-
The miR27b-CCNG1-P53-miR-508-5p axis regulates multidrug resistance of gastric cancer.Oncotarget. 2016 Jan 5;7(1):538-49. doi: 10.18632/oncotarget.6374. Oncotarget. 2016. PMID: 26623719 Free PMC article.
-
β-Elemene inhibits the metastasis of multidrug-resistant gastric cancer cells through miR-1323/Cbl-b/EGFR pathway.Phytomedicine. 2020 Apr;69:153184. doi: 10.1016/j.phymed.2020.153184. Epub 2020 Feb 10. Phytomedicine. 2020. PMID: 32199253
-
Long non-coding RNA CASC9 promotes the progression and development of gastric cancer via regulating miR-370/EGFR axis.Dig Liver Dis. 2021 Apr;53(4):509-516. doi: 10.1016/j.dld.2020.12.115. Epub 2021 Jan 18. Dig Liver Dis. 2021. PMID: 33478874
Cited by
-
An Anticancer Bioactive Peptide Combined with Oxaliplatin Inhibited Gastric Cancer Cells In vitro and In vivo.Curr Protein Pept Sci. 2025;26(6):493-510. doi: 10.2174/0113892037350632241205040150. Curr Protein Pept Sci. 2025. PMID: 39791151
-
Evaluating H2BC9 as a potential diagnostic and prognostic biomarker in head and neck squamous cell carcinoma.Eur J Med Res. 2025 Jan 27;30(1):54. doi: 10.1186/s40001-025-02301-3. Eur J Med Res. 2025. PMID: 39865289 Free PMC article.
-
Artificial intelligence-driven microRNA signature for early detection of gastric cancer: discovery and clinical functional exploration.Br J Cancer. 2025 Jun;132(10):957-972. doi: 10.1038/s41416-025-02984-9. Epub 2025 Apr 15. Br J Cancer. 2025. PMID: 40234666
References
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. - PubMed
-
- Cunningham D., Allum W.H., Stenning S.P., Thompson J.N., Van de Velde C.J., Nicolson M., et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006;355:11–20. - PubMed
-
- Huang H., Yang X.J., Gao R. Research advances in the mechanisms of gastric cancer multidrug resistance. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2016;38:739–745. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

