Neuroprotective efficacy of the glucocorticoid receptor modulator PT150 in the rotenone mouse model of Parkinson's disease
- PMID: 38960072
- PMCID: PMC11796432
- DOI: 10.1016/j.neuro.2024.06.017
Neuroprotective efficacy of the glucocorticoid receptor modulator PT150 in the rotenone mouse model of Parkinson's disease
Abstract
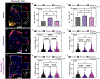
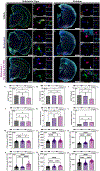
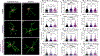
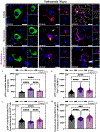
Parkinson's disease (PD) is the most common neurodegenerative movement disorder worldwide. Current treatments for PD largely center around dopamine replacement therapies and fail to prevent the progression of pathology, underscoring the need for neuroprotective interventions. Approaches that target neuroinflammation, which occurs prior to dopaminergic neuron (DAn) loss in the substantia nigra (SN), represent a promising therapeutic strategy. The glucocorticoid receptor (GR) has been implicated in the neuropathology of PD and modulates numerous neuroinflammatory signaling pathways in the brain. Therefore, we investigated the neuroprotective effects of the novel GR modulator, PT150, in the rotenone mouse model of PD, postulating that inhibition of glial inflammation would protect DAn and reduce accumulation of neurotoxic misfolded ⍺-synuclein protein. C57Bl/6 mice were exposed to 2.5 mg/kg/day rotenone by intraperitoneal injection for 14 days. Upon completion of rotenone dosing, mice were orally treated at day 15 with 30 mg/kg/day or 100 mg/kg/day PT150 in the 14-day post-lesioning incubation period, during which the majority of DAn loss and α-synuclein (α-syn) accumulation occurs. Our results indicate that treatment with PT150 reduced both loss of DAn and microgliosis in the nigrostriatal pathway. Although morphologic features of astrogliosis were not attenuated, PT150 treatment promoted potentially neuroprotective activity in these cells, including increased phagocytosis of hyperphosphorylated α-syn. Ultimately, PT150 treatment reduced the loss of DAn cell bodies in the SN, but not the striatum, and prohibited intra-neuronal accumulation of α-syn. Together, these data indicate that PT150 effectively reduced SN pathology in the rotenone mouse model of PD.
Keywords: Astrocyte; Microglia; Molecular modeling; Neurodegeneration; Neuroinflammation; Neuroprotection; Parkinson's disease.
Copyright © 2024. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Ronald Tjalkens reports financial support was provided by Colorado State University. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Update of
-
Neuroprotective Efficacy of the Glucocorticoid Receptor Modulator PT150 in the Rotenone Mouse Model of Parkinson's Disease.bioRxiv [Preprint]. 2024 Apr 15:2024.04.12.589261. doi: 10.1101/2024.04.12.589261. bioRxiv. 2024. Update in: Neurotoxicology. 2024 Jul;103:320-334. doi: 10.1016/j.neuro.2024.06.017. PMID: 38659796 Free PMC article. Updated. Preprint.
Similar articles
-
Neuroprotective Efficacy of the Glucocorticoid Receptor Modulator PT150 in the Rotenone Mouse Model of Parkinson's Disease.bioRxiv [Preprint]. 2024 Apr 15:2024.04.12.589261. doi: 10.1101/2024.04.12.589261. bioRxiv. 2024. Update in: Neurotoxicology. 2024 Jul;103:320-334. doi: 10.1016/j.neuro.2024.06.017. PMID: 38659796 Free PMC article. Updated. Preprint.
-
Rotenone induces regionally distinct α-synuclein protein aggregation and activation of glia prior to loss of dopaminergic neurons in C57Bl/6 mice.Neurobiol Dis. 2022 Jun 1;167:105685. doi: 10.1016/j.nbd.2022.105685. Epub 2022 Mar 5. Neurobiol Dis. 2022. PMID: 35257879 Free PMC article.
-
GPR40 agonist ameliorates neurodegeneration and motor impairment by regulating NLRP3 inflammasome in Parkinson's disease animal models.Pharmacol Res. 2024 Nov;209:107432. doi: 10.1016/j.phrs.2024.107432. Epub 2024 Sep 21. Pharmacol Res. 2024. PMID: 39313081
-
Calpain activation and progression of inflammatory cycles in Parkinson's disease.Front Biosci (Landmark Ed). 2022 Jan 13;27(1):20. doi: 10.31083/j.fbl2701020. Front Biosci (Landmark Ed). 2022. PMID: 35090325 Free PMC article. Review.
-
Emerging Treatment Approaches for Parkinson's Disease.Front Neurosci. 2018 Oct 8;12:693. doi: 10.3389/fnins.2018.00693. eCollection 2018. Front Neurosci. 2018. PMID: 30349448 Free PMC article. Review.
Cited by
-
Rotenone inhibited osteosarcoma metastasis by modulating ZO-2 expression and location via the ROS/Ca2+/AMPK pathway.Redox Rep. 2025 Dec;30(1):2493556. doi: 10.1080/13510002.2025.2493556. Epub 2025 Apr 17. Redox Rep. 2025. PMID: 40247635 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

