Molecular tumor board: molecularly adjusted therapy upon identification and functional validation of a novel ALK resistance mutation in a case of lung adenocarcinoma
- PMID: 38960389
- PMCID: PMC11783293
- DOI: 10.1093/oncolo/oyae143
Molecular tumor board: molecularly adjusted therapy upon identification and functional validation of a novel ALK resistance mutation in a case of lung adenocarcinoma
Abstract
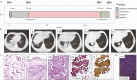
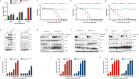
We report a case of a long-term surviving patient with EML4/ALK translocated non-small cell adenocarcinoma of the lung in UICC8 stage IVA. During recurrence under continuous crizotinib therapy, a hitherto insufficiently characterized missense mutation in the ALK gene (Arg1181His) was identified through targeted sequencing. The aforementioned EML4/ALK translocation could still be detected in this situation. Employing a 3D reconstruction of the ALK tertiary structure, considering its interaction with various ALK inhibitors at the molecular binding site, our analysis indicated the presence of a mutation associated with crizotinib resistance. To validate the biological relevance of this previously unknown mutation, we carried out an in vitro validation approach in cell culture in addition to the molecular diagnostics accompanied by the molecular tumor board. The tumor scenario was mimicked through retroviral transfection. Our comparative in vitro treatment regimen paired with the clinical trajectory of the patient, corroborated our initial clinical and biochemical suspicions. Our approach demonstrates preclinical, in silico, and clinical evidence of a novel crizotinib resistance mutation in ALK as well as sensitivity toward brigatinib and potentially lorlatinib. In future cases, this procedure represents an important contribution to functional diagnostics in the context of molecular tumor boards.
Keywords: ALK inhibitors; ALK translocations; NSCLC; functional validation; molecular profiling.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
N.G. received travel support from Janssen, BeiGene, and Roche and serves as an advisor to Roche, AstraZeneca, Takeda, Janssen, Incyte, and Stemline. M.W.G. received honoraria from BeiGene, travel support from Sobi, and serves as an advisor for AstraZeneca and Novartis. K.S. received honoraria and serves as an advisor to AstraZeneca, BMS, MSD, Sanofi, and Boehringer Ingelheim. H.M.W. received honoraria from BeiGene and Viatris as well as travel support from Janssen. The other authors indicated no financial relationships.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

