Same-visit hepatitis C testing and treatment to accelerate cure among people who inject drugs (the QuickStart Study): a cluster randomised cross-over trial protocol
- PMID: 38960465
- PMCID: PMC11227801
- DOI: 10.1136/bmjopen-2023-083502
Same-visit hepatitis C testing and treatment to accelerate cure among people who inject drugs (the QuickStart Study): a cluster randomised cross-over trial protocol
Abstract
Introduction: Despite universal access to government-funded direct-acting antivirals (DAAs) in 2016, the rate of hepatitis C treatment uptake in Australia has declined substantially. Most hepatitis C is related to injecting drug use; reducing the hepatitis C burden among people who inject drugs (PWID) is, therefore, paramount to reach hepatitis C elimination targets. Increasing DAA uptake by PWID is important for interrupting transmission and reducing incidence, as well as reducing morbidity and mortality and improving quality of life of PWID and meeting Australia's hepatitis C elimination targets.
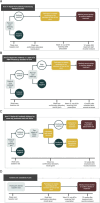
Methods and analysis: A cluster randomised cross-over trial will be conducted with three intervention arms and a control arm. Arm A will receive rapid hepatitis C virus (HCV) antibody testing; arm B will receive rapid HCV antibody and rapid RNA testing; arm C will receive rapid HCV antibody testing and same-day treatment initiation for HCV antibody-positive participants; the control arm will receive standard of care. The primary outcomes will be (a) the proportion of participants with HCV commencing treatment and (b) the proportion of participants with HCV achieving cure. Analyses will be conducted on an intention-to-treat basis with mixed-effects logistic regression models.
Ethics and dissemination: The study has been approved by the Alfred Ethics Committee (number HREC/64731/Alfred-2020-217547). Each participant will provide written informed consent. Reportable adverse events will be reported to the reviewing ethics committee. The findings will be presented at scientific conferences and published in peer-reviewed journals.
Trial registration number: NCT05016609.
Trial progression: The study commenced recruitment on 9 March 2022 and is expected to complete recruitment in December 2024.
Keywords: Hepatology; INFECTIOUS DISEASES; Public health.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: JSD, MH and AP report investigator-iniated funding to their institution from Gilead Sciences, AbbVie and Merck. JSD reports honoraria to his institution from Gilead Sciences and AbbVie.
Figures
Similar articles
-
Reaching mEthadone users Attending Community pHarmacies with HCV: an international cluster randomised controlled trial protocol (REACH HCV).BMJ Open. 2020 Aug 30;10(8):e036501. doi: 10.1136/bmjopen-2019-036501. BMJ Open. 2020. PMID: 32868356 Free PMC article.
-
Opportunistic treatment of hepatitis C virus infection (OPPORTUNI-C): study protocol for a pragmatic stepped wedge cluster randomized trial of immediate versus outpatient treatment initiation among hospitalized people who inject drugs.Trials. 2020 Jun 15;21(1):524. doi: 10.1186/s13063-020-04434-8. Trials. 2020. PMID: 32539853 Free PMC article.
-
Towards HCV elimination among people who inject drugs in Hai Phong, Vietnam: study protocol for an effectiveness-implementation trial evaluating an integrated model of HCV care (DRIVE-C: DRug use & Infections in ViEtnam-hepatitis C).BMJ Open. 2020 Nov 18;10(11):e039234. doi: 10.1136/bmjopen-2020-039234. BMJ Open. 2020. PMID: 33208326 Free PMC article.
-
Interventions to enhance testing and linkage to treatment for hepatitis C infection for people who inject drugs: A systematic review and meta-analysis.Int J Drug Policy. 2023 Jan;111:103917. doi: 10.1016/j.drugpo.2022.103917. Epub 2022 Dec 19. Int J Drug Policy. 2023. PMID: 36542883
-
Management of hepatitis C virus/HIV coinfection among people who use drugs in the era of direct-acting antiviral-based therapy.Clin Infect Dis. 2013 Aug;57 Suppl 2(Suppl 2):S118-24. doi: 10.1093/cid/cit326. Clin Infect Dis. 2013. PMID: 23884059 Free PMC article. Review.
Cited by
-
Balancing Efficiency and Accuracy in Hepatitis C Rapid Antibody Testing: Insights From a Cluster Randomised Crossover Trial.J Viral Hepat. 2025 Aug;32(8):e70043. doi: 10.1111/jvh.70043. J Viral Hepat. 2025. PMID: 40607684 Free PMC article. Clinical Trial.
References
-
- World Health Organization . Hepatitis C: fact sheet 2022. Available: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c [Accessed 23 Jun 2022].
-
- Burnet Institute . Australia’s progress towards hepatitis C elimination: annual report 2021, 2021. Available: https://burnet.edu.au/system/asset/file/5001/BurnetKirby-hepC-2021-repor...
-
- Grove A. The pharmaceutical benefits scheme: a quick guide 2018. Available: https://www.aph.gov.au/About_Parliament/Parliamentary_Departments/Parlia... [Accessed 15 Dec 2022].
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
