Patient-Reported Outcomes in COVID-19 Treatment with Monoclonal Antibodies Reveal Benefits in Return to Usual Activities
- PMID: 38961047
- PMCID: PMC11266324
- DOI: 10.1007/s40121-024-01013-1
Patient-Reported Outcomes in COVID-19 Treatment with Monoclonal Antibodies Reveal Benefits in Return to Usual Activities
Abstract
Introduction: This study aimed to assess the effects of a monoclonal antibody (mAb) combination on symptoms, daily function, and overall health-related quality of life.
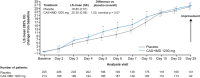
Methods: We analyzed patient-reported outcomes data from symptomatic outpatients in a phase 1/2/3 trial. Patients with confirmed SARS-CoV-2 infection and ≥ 1 risk factor for severe COVID-19 received mAb treatment (casirivimab plus imdevimab 1200 mg) or placebo. Prespecified exploratory assessments included time to sustained symptoms resolution, usual health, and return to usual activities (assessed daily for 29 days). The trial was conducted from September 2020 to February 2021, prior to widespread COVID-19 vaccination programs and Omicron-lineage variants against which casirivimab + imdevimab is not active.
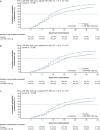
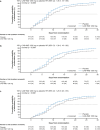
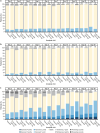
Results: In this analysis 736 outpatients received mAb and 1341 received placebo. Median time to sustained symptoms resolution was consistently shorter with mAb versus placebo (≥ 2 consecutive days: 14 vs 17 days, [nominal p = 0.0017]; ≥ 3 consecutive days: 17 vs 21 days, [nominal p = 0.0046]). Median time to sustained return to usual health and usual activities were both consistently shorter with mAb versus placebo (≥ 2 consecutive days: 12 vs 15 days [nominal p = 0.0001] and 9 vs 11 days [nominal p = 0.0001], respectively; ≥ 3 consecutive days: 14 vs 18 days [nominal p = 0.0003] and 10 vs 13 days [nominal p = 0.0041], respectively).
Conclusions: mAb treatment against susceptible SARS-CoV-2 strains improved how patients feel and function, as evidenced by shortened time to sustained symptoms resolution and return to usual health and activities. Future studies are warranted to assess the patient experience with next generation mAbs.
Clinicaltrials: GOV: Registration number, NCT04425629; Submission date June 11, 2020.
Keywords: COVID-19; Monoclonal antibodies; Patient-reported outcomes; Quality of life; SARS-CoV-2; Symptoms.
© 2024. The Author(s).
Conflict of interest statement
Diana Rofail is an employee/stockholder of Regeneron Pharmaceuticals, Inc. and former employee and stockholder of F. Hoffmann-La Roche AG. Mohamed Hussein, Thomas Norton, Shazia Ali, Vera Mastey, Boaz Hirshberg, and Gregory P. Geba are employees/stockholders of Regeneron Pharmaceuticals, Inc. Ulrike Naumann and Cristina Ivanescu are employees of IQVIA and received consultancy fees from Regeneron Pharmaceuticals, Inc. Anna J. Podolanczuk received consultancy fees from Regeneron Pharmaceuticals, Inc., received honoraria from NACE (CME), and has participated in an advisory board for Boehringer Ingelheim.
Figures


Similar articles
-
Effect of timing of casirivimab and imdevimab administration relative to mRNA-1273 COVID-19 vaccination on vaccine-induced SARS-CoV-2 neutralising antibody responses: a prospective, open-label, phase 2, randomised controlled trial.Lancet Infect Dis. 2025 Jan;25(1):52-67. doi: 10.1016/S1473-3099(24)00421-3. Epub 2024 Sep 2. Lancet Infect Dis. 2025. PMID: 39236733 Clinical Trial.
-
Efficacy and safety of a single dose of casirivimab and imdevimab for the prevention of COVID-19 over an 8-month period: a randomised, double-blind, placebo-controlled trial.Lancet Infect Dis. 2022 Oct;22(10):1444-1454. doi: 10.1016/S1473-3099(22)00416-9. Epub 2022 Jul 5. Lancet Infect Dis. 2022. PMID: 35803290 Free PMC article. Clinical Trial.
-
The UPMC OPTIMISE-C19 (OPtimizing Treatment and Impact of Monoclonal antIbodieS through Evaluation for COVID-19) trial: a structured summary of a study protocol for an open-label, pragmatic, comparative effectiveness platform trial with response-adaptive randomization.Trials. 2021 May 25;22(1):363. doi: 10.1186/s13063-021-05316-3. Trials. 2021. PMID: 34034784 Free PMC article.
-
Susceptibility of SARS-CoV-2 Omicron Variants to Therapeutic Monoclonal Antibodies: Systematic Review and Meta-analysis.Microbiol Spectr. 2022 Aug 31;10(4):e0092622. doi: 10.1128/spectrum.00926-22. Epub 2022 Jun 14. Microbiol Spectr. 2022. PMID: 35700134 Free PMC article.
-
Outpatient Treatment of Confirmed COVID-19: A Living, Rapid Review for the American College of Physicians.Ann Intern Med. 2023 Jan;176(1):92-104. doi: 10.7326/M22-2202. Epub 2022 Nov 29. Ann Intern Med. 2023. Update in: Ann Intern Med. 2023 Oct;176(10):1377-1385. doi: 10.7326/M23-1626. PMID: 36442056 Free PMC article. Updated. Review.
References
-
- World Health Organization. WHO coronavirus (COVID-19) dashboard. 2024. https://covid19.who.int/. Accessed 17 Jan 2024.
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

