Three SARS-CoV-2 spike protein variants delivered intranasally by measles and mumps vaccines are broadly protective
- PMID: 38961063
- PMCID: PMC11222507
- DOI: 10.1038/s41467-024-49443-2
Three SARS-CoV-2 spike protein variants delivered intranasally by measles and mumps vaccines are broadly protective
Abstract
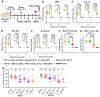
As the new SARS-CoV-2 Omicron variants and subvariants emerge, there is an urgency to develop intranasal, broadly protective vaccines. Here, we developed highly efficacious, intranasal trivalent SARS-CoV-2 vaccine candidates (TVC) based on three components of the MMR vaccine: measles virus (MeV), mumps virus (MuV) Jeryl Lynn (JL1) strain, and MuV JL2 strain. Specifically, MeV, MuV-JL1, and MuV-JL2 vaccine strains, each expressing prefusion spike (preS-6P) from a different variant of concern (VoC), were combined to generate TVCs. Intranasal immunization of IFNAR1-/- mice and female hamsters with TVCs generated high levels of S-specific serum IgG antibodies, broad neutralizing antibodies, and mucosal IgA antibodies as well as tissue-resident memory T cells in the lungs. The immunized female hamsters were protected from challenge with SARS-CoV-2 original WA1, B.1.617.2, and B.1.1.529 strains. The preexisting MeV and MuV immunity does not significantly interfere with the efficacy of TVC. Thus, the trivalent platform is a promising next-generation SARS-CoV-2 vaccine candidate.
© 2024. The Author(s).
Conflict of interest statement
The Ohio State University has filed an invention report on this approach to the development of intranasal trivalent SARS-CoV-2 vaccines. J.L., Y.Z., J.X., M.C. and X.L. are the inventors. Other authors do not have competing interests.
Figures




References
MeSH terms
Substances
Supplementary concepts
Grants and funding
- AI093848/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- AI112524/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01 AI090060/AI/NIAID NIH HHS/United States
- U54CA260582/Division of Cancer Prevention, National Cancer Institute (NCI Division of Cancer Prevention)
- R01 AI093848/AI/NIAID NIH HHS/United States
- P01 AI175399/AI/NIAID NIH HHS/United States
- P01 AI112524/AI/NIAID NIH HHS/United States
- R01 AI157205/AI/NIAID NIH HHS/United States
- AI090060/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01 AI145144/AI/NIAID NIH HHS/United States
- R21 AI180667/AI/NIAID NIH HHS/United States
- T32AI165391/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- AI42733/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- AI157205/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- U19 AI142733/AI/NIAID NIH HHS/United States
- U54 CA260582/CA/NCI NIH HHS/United States
- U54CA260582/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- R01 HL168501/HL/NHLBI NIH HHS/United States
- AI145144/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- AI175399/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- T32 AI165391/AI/NIAID NIH HHS/United States
- R01 AI130110/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

