Anti-CCL2 therapy reduces oxygen toxicity to the immature lung
- PMID: 38961074
- PMCID: PMC11222519
- DOI: 10.1038/s41420-024-02073-5
Anti-CCL2 therapy reduces oxygen toxicity to the immature lung
Abstract
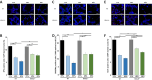
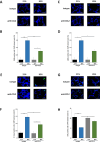
Oxygen toxicity constitutes a key contributor to bronchopulmonary dysplasia (BPD). Critical step in the pathogenesis of BPD is the inflammatory response in the immature lung with the release of pro-inflammatory cytokines and the influx of innate immune cells. Identification of efficient therapies to alleviate the inflammatory response remains an unmet research priority. First, we studied macrophage and neutrophil profiles in tracheal aspirates of n = 103 preterm infants <29 weeks´ gestation requiring mechanical ventilation. While no differences were present at birth, a higher fraction of macrophages, the predominance of the CD14+CD16+ subtype on day 5 of life was associated with moderate/severe BPD. Newborn CCL-2-/- mice insufficient in pulmonary macrophage recruitment had a reduced influx of neutrophils, lower apoptosis induction in the pulmonary tissue and better-preserved lung morphometry with higher counts of type II cells, mesenchymal stem cells and vascular endothelial cells when exposed to hyperoxia for 7 days. To study the benefit of a targeted approach to prevent the pulmonary influx of macrophages, wildtype mice were repeatedly treated with CCL-2 blocking antibodies while exposed to hyperoxia for 7 days. Congruent with the results in CCL-2-/- animals, the therapeutic intervention reduced the pulmonary inflammatory response, attenuated cell death in the lung tissue and better-preserved lung morphometry. Overall, our preclinical and clinical datasets document the predominant role of macrophage recruitment to the pathogenesis of BPD and establish the abrogation of CCL-2 function as novel approach to protect the immature lung from hyperoxic injury.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
TRAIL protects the immature lung from hyperoxic injury.Cell Death Dis. 2022 Jul 15;13(7):614. doi: 10.1038/s41419-022-05072-5. Cell Death Dis. 2022. PMID: 35840556 Free PMC article.
-
[Anti-inflammatory effects of erythropoietin on hyperoxia-induced bronchopulmonary dysplasia in newborn rats].Zhonghua Er Ke Za Zhi. 2009 Jun;47(6):446-51. Zhonghua Er Ke Za Zhi. 2009. PMID: 19951473 Chinese.
-
Comparative Effects of Bone Marrow-derived Versus Umbilical Cord Tissue Mesenchymal Stem Cells in an Experimental Model of Bronchopulmonary Dysplasia.Stem Cells Transl Med. 2022 Mar 17;11(2):189-199. doi: 10.1093/stcltm/szab011. Stem Cells Transl Med. 2022. PMID: 35298658 Free PMC article.
-
Surfactant protein D and bronchopulmonary dysplasia: a new way to approach an old problem.Respir Res. 2021 May 8;22(1):141. doi: 10.1186/s12931-021-01738-4. Respir Res. 2021. PMID: 33964929 Free PMC article. Review.
-
Inhalation or instillation of steroids for the prevention of bronchopulmonary dysplasia.Neonatology. 2015;107(4):358-9. doi: 10.1159/000381132. Epub 2015 Jun 5. Neonatology. 2015. PMID: 26044104 Review.
Cited by
-
The MFGE8/integrin β3 axis mitigates experimental neutrophilic asthma by suppressing NLRP3-Caspase-1 pathway-mediated NETosis.Respir Res. 2025 Jul 2;26(1):229. doi: 10.1186/s12931-025-03313-7. Respir Res. 2025. PMID: 40605028 Free PMC article.
-
Melatonin Activates KEAP1/NRF2/PTGS2 Pathway to Attenuate Hyperoxia-Driven Ferroptosis in Bronchopulmonary Dysplasia.J Inflamm Res. 2025 Jun 11;18:7571-7583. doi: 10.2147/JIR.S520404. eCollection 2025. J Inflamm Res. 2025. PMID: 40524969 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

