Flotillin-mediated stabilization of unfolded proteins in bacterial membrane microdomains
- PMID: 38961085
- PMCID: PMC11222466
- DOI: 10.1038/s41467-024-49951-1
Flotillin-mediated stabilization of unfolded proteins in bacterial membrane microdomains
Abstract
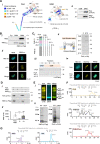
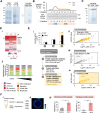
The function of many bacterial processes depends on the formation of functional membrane microdomains (FMMs), which resemble the lipid rafts of eukaryotic cells. However, the mechanism and the biological function of these membrane microdomains remain unclear. Here, we show that FMMs in the pathogen methicillin-resistant Staphylococcus aureus (MRSA) are dedicated to confining and stabilizing proteins unfolded due to cellular stress. The FMM scaffold protein flotillin forms a clamp-shaped oligomer that holds unfolded proteins, stabilizing them and favoring their correct folding. This process does not impose a direct energy cost on the cell and is crucial to survival of ATP-depleted bacteria, and thus to pathogenesis. Consequently, FMM disassembling causes the accumulation of unfolded proteins, which compromise MRSA viability during infection and cause penicillin re-sensitization due to PBP2a unfolding. Thus, our results indicate that FMMs mediate ATP-independent stabilization of unfolded proteins, which is essential for bacterial viability during infection.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interest
Figures




Similar articles
-
Membrane Microdomain Disassembly Inhibits MRSA Antibiotic Resistance.Cell. 2017 Nov 30;171(6):1354-1367.e20. doi: 10.1016/j.cell.2017.10.012. Epub 2017 Nov 2. Cell. 2017. PMID: 29103614 Free PMC article.
-
Flotillin scaffold activity contributes to type VII secretion system assembly in Staphylococcus aureus.PLoS Pathog. 2017 Nov 22;13(11):e1006728. doi: 10.1371/journal.ppat.1006728. eCollection 2017 Nov. PLoS Pathog. 2017. PMID: 29166667 Free PMC article.
-
Simulation study of domain formation in a model bacterial membrane.Phys Chem Chem Phys. 2022 Aug 3;24(30):18133-18143. doi: 10.1039/d2cp01873j. Phys Chem Chem Phys. 2022. PMID: 35856570
-
Clinical, Epidemiologic, and Laboratory Aspects of Methicillin-Resistant Staphylococcus aureus Infections.Methods Mol Biol. 2020;2069:1-28. doi: 10.1007/978-1-4939-9849-4_1. Methods Mol Biol. 2020. PMID: 31523762 Review.
-
A Review on Five and Six-Membered Heterocyclic Compounds Targeting the Penicillin-Binding Protein 2 (PBP2A) of Methicillin-Resistant Staphylococcus aureus (MRSA).Molecules. 2023 Oct 10;28(20):7008. doi: 10.3390/molecules28207008. Molecules. 2023. PMID: 37894491 Free PMC article. Review.
Cited by
-
Flotillins in membrane trafficking and physiopathology.Biol Cell. 2025 Jan;117(1):e2400134. doi: 10.1111/boc.202400134. Biol Cell. 2025. PMID: 39877933 Free PMC article. Review.
-
Antechodynamics and Antechokinetics: Dynamics and Kinetics of Antibiotic Resistance Biomolecules.Biomolecules. 2025 Jun 5;15(6):823. doi: 10.3390/biom15060823. Biomolecules. 2025. PMID: 40563463 Free PMC article. Review.
-
Structural Plasticity of the Membrane-Bound Protein Degradation Assembly Supports Bacterial Adaptation to Stress.bioRxiv [Preprint]. 2025 Jul 25:2025.07.21.662073. doi: 10.1101/2025.07.21.662073. bioRxiv. 2025. PMID: 40777504 Free PMC article. Preprint.
-
Toxic Effects of Butanol in the Plane of the Cell Membrane.Langmuir. 2025 Jan 21;41(2):1281-1296. doi: 10.1021/acs.langmuir.4c03677. Epub 2025 Jan 8. Langmuir. 2025. PMID: 39772768
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

