Undenatured type II collagen protects against collagen-induced arthritis by restoring gut-joint homeostasis and immunity
- PMID: 38961129
- PMCID: PMC11222443
- DOI: 10.1038/s42003-024-06476-z
Undenatured type II collagen protects against collagen-induced arthritis by restoring gut-joint homeostasis and immunity
Abstract
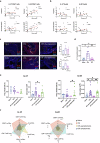
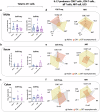
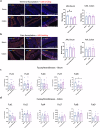
Oral administration of harmless antigens can induce suppression of reactive immune responses, a process that capitalises on the ability of the gastrointestinal tract to tolerate exposure to food and commensal microbiome without triggering inflammatory responses. Repeating exposure to type II collagen induces oral tolerance and inhibits induction of arthritis, a chronic inflammatory joint condition. Although some mechanisms underlying oral tolerance are described, how dysregulation of gut immune networks impacts on inflammation of distant tissues like the joints is unclear. We used undenatured type II collagen in a prophylactic regime -7.33 mg/kg three times/week- to describe the mechanisms associated with protective oral immune-therapy (OIT) in gut and joint during experimental Collagen-Induced Arthritis (CIA). OIT reduced disease incidence to 50%, with reduced expression of IL-17 and IL-22 in the joints of asymptomatic mice. Moreover, whilst the gut tissue of arthritic mice shows substantial damage and activation of tissue-specific immune networks, oral administration of undenatured type II collagen protects against gut pathology in all mice, symptomatic and asymptomatic, rewiring IL-17/IL-22 networks. Furthermore, gut fucosylation and microbiome composition were also modulated. These results corroborate the relevance of the gut-joint axis in arthritis, showing novel regulatory mechanisms linked to therapeutic OIT in joint disease.
© 2024. The Author(s).
Conflict of interest statement
Z.S., E.K.A. and A.S. are employees of Lonza Greenwood LLC., Greenwood, SC, USA. The remaining authors declare no competing interests.
Figures






Similar articles
-
Novel therapeutic compound tuftsin-phosphorylcholine attenuates collagen-induced arthritis.Clin Exp Immunol. 2016 Apr;184(1):19-28. doi: 10.1111/cei.12745. Epub 2016 Feb 4. Clin Exp Immunol. 2016. PMID: 26618631 Free PMC article.
-
Water-soluble undenatured type II collagen ameliorates collagen-induced arthritis in mice.J Med Food. 2013 Nov;16(11):1039-45. doi: 10.1089/jmf.2013.2911. Epub 2013 Oct 31. J Med Food. 2013. PMID: 24175655
-
Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis.Arthritis Res. 1999;1(1):81-91. doi: 10.1186/ar14. Epub 1999 Oct 26. Arthritis Res. 1999. PMID: 11056663 Free PMC article.
-
Oral administration of type-II collagen suppresses IL-17-associated RANKL expression of CD4+ T cells in collagen-induced arthritis.Immunol Lett. 2008 Apr 15;117(1):16-25. doi: 10.1016/j.imlet.2007.09.011. Epub 2007 Oct 22. Immunol Lett. 2008. PMID: 18242716
-
Type II collagen oral tolerance; mechanism and role in collagen-induced arthritis and rheumatoid arthritis.Mod Rheumatol. 2009;19(6):581-9. doi: 10.1007/s10165-009-0210-0. Epub 2009 Aug 21. Mod Rheumatol. 2009. PMID: 19697097 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

