PSA doubling time 4.65 months as an optimal cut-off of Japanese nonmetastatic castration-resistant prostate cancer
- PMID: 38961131
- PMCID: PMC11222484
- DOI: 10.1038/s41598-024-65969-3
PSA doubling time 4.65 months as an optimal cut-off of Japanese nonmetastatic castration-resistant prostate cancer
Abstract
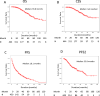
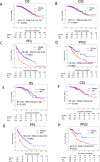
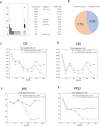
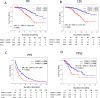
A multicenter study of nonmetastatic castration-resistant prostate cancer (nmCRPC) was conducted to identify the optimal cut-off value of prostate-specific antigen (PSA) doubling time (PSADT) that correlated with the prognosis in Japanese nmCRPC. Of the 515 patients diagnosed and treated for nmCRPC at 25 participating Japanese Urological Oncology Group centers, 450 patients with complete clinical information were included. The prognostic values of clinical factors were evaluated with respect to prostate specific antigen progression-free (PFS), cancer-specific survival (CSS), and overall survival (OS). The optimal cutoff value of PSADT was identified using survival tree analysis by Python. The Median PSA and PSADT at diagnosis of nmCRPC were 3.3 ng/ml, and 5.2 months, respectively. Patients treated with novel hormonal therapy (NHT) showed significantly longer PFS (HR: hazard ratio 0.38, p < 0.0001) and PFS2 (HR 0.45, p < 0.0001) than those treated with vintage nonsteroidal antiandrogen agent (Vintage). The survival tree identified 4.65 months as the most prognostic PSADT cutoff point. Among the clinical and pathological factors PSADT of < 4.65 months remained an independent prognostic factor for OS (HR 2.96, p = 0.0003) and CSS (HR 3.66, p < 0.0001). Current data represented optimal cut-off of PSADT 4.65 months for a Japanese nmCRPC.
Keywords: NHT; PSA doubling time; Prostate cancer; Vintage; nmCRPC.
© 2024. The Author(s).
Conflict of interest statement
Shinichi Sakamoto received honoraria from Janssen Inc. Shintaro Narita received honoraria from Janssen, and AstraZeneca Inc. Masaki Shiota received honoraria from Janssen, AstraZeneca, Astellas, Sanofi, and Bayer Inc. and research funding support from Daiichi Sankyo Inc. Takahiro Kimura received honoraria from Astellas, AstraZeneca, Bayer, Janssen, Sanofi, and Takeda Inc. Hiroshi Kitamura received honoraria from Astellas, AstraZeneca, Janssen, Sanofi, and Takeda Inc. and research grant from AstraZeneca Inc. Kohei Hashimoto received honoraria from Astellas, Janssen, Bayer and AstraZeneca Inc. Naoki Terada received honoraria from Janssen, Astellas and Bayer Inc. Hideaki Miyake received honoraria from Astellas, Bayer, Janssen, Sanofi, and Takeda Inc. Akira Joraku, Eiryo Kawakami, Jun Miki, Kodai Sato, Naotaka Nishiyama, Ryotaro Tomida, Ryuji Matsumoto, Shigetaka Suekane, Shuichi Tatarano, Takayuki Yoshino, Takuma Kato, Tomohiko Ichikawa, Tomoyuki Kaneko, Toshihiro Saito, Yoshiyuki Matsui, Yuko Yoshio, Yusuke Shiraishi have no conflict of interest to disclose.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

