Influenza A virus during pregnancy disrupts maternal intestinal immunity and fetal cortical development in a dose- and time-dependent manner
- PMID: 38961232
- PMCID: PMC11649561
- DOI: 10.1038/s41380-024-02648-9
Influenza A virus during pregnancy disrupts maternal intestinal immunity and fetal cortical development in a dose- and time-dependent manner
Abstract
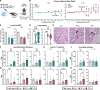
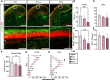
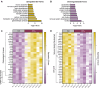
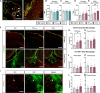
Epidemiological studies link exposure to viral infection during pregnancy, including influenza A virus (IAV) infection, with increased incidence of neurodevelopmental disorders (NDDs) in offspring. Models of maternal immune activation (MIA) using viral mimetics demonstrate that activation of maternal intestinal T helper 17 (TH17) cells, which produce effector cytokine interleukin (IL)-17, leads to aberrant fetal brain development, such as neocortical malformations. Fetal microglia and border-associated macrophages (BAMs) also serve as potential cellular mediators of MIA-induced cortical abnormalities. However, neither the inflammation-induced TH17 cell pathway nor fetal brain-resident macrophages have been thoroughly examined in models of live viral infection during pregnancy. Here, we inoculated pregnant mice with two infectious doses of IAV and evaluated peak innate and adaptive immune responses in the dam and fetus. While respiratory IAV infection led to dose-dependent maternal colonic shortening and microbial dysregulation, there was no elevation in intestinal TH17 cells nor IL-17. Systemically, IAV resulted in consistent dose- and time-dependent increases in IL-6 and IFN-γ. Fetal cortical abnormalities and global changes in fetal brain transcripts were observable in the high-but not the moderate-dose IAV group. Profiling of fetal microglia and BAMs revealed dose- and time-dependent differences in the numbers of meningeal but not choroid plexus BAMs, while microglial numbers and proliferative capacity of Iba1+ cells remained constant. Fetal brain-resident macrophages increased phagocytic CD68 expression, also in a dose- and time-dependent fashion. Taken together, our findings indicate that certain features of MIA are conserved between mimetic and live virus models, while others are not. Overall, we provide consistent evidence of an infection severity threshold for downstream maternal inflammation and fetal cortical abnormalities, which recapitulates a key feature of the epidemiological data and further underscores the importance of using live pathogens in NDD modeling to better evaluate the complete immune response and to improve translation to the clinic.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Brain region-specific alterations in gene expression trajectories in the offspring born from influenza A virus infected mice.Brain Behav Immun. 2024 Aug;120:488-498. doi: 10.1016/j.bbi.2024.06.025. Epub 2024 Jun 24. Brain Behav Immun. 2024. PMID: 38925418
-
Microglia, the missing link in maternal immune activation and fetal neurodevelopment; and a possible link in preeclampsia and disturbed neurodevelopment?J Reprod Immunol. 2018 Apr;126:18-22. doi: 10.1016/j.jri.2018.01.004. Epub 2018 Jan 31. J Reprod Immunol. 2018. PMID: 29421625 Review.
-
At the crux of maternal immune activation: Viruses, microglia, microbes, and IL-17A.Immunol Rev. 2022 Oct;311(1):205-223. doi: 10.1111/imr.13125. Epub 2022 Aug 18. Immunol Rev. 2022. PMID: 35979731 Free PMC article. Review.
-
Moderately pathogenic maternal influenza A virus infection disrupts placental integrity but spares the fetal brain.Brain Behav Immun. 2021 Aug;96:28-39. doi: 10.1016/j.bbi.2021.05.004. Epub 2021 May 12. Brain Behav Immun. 2021. PMID: 33989741 Free PMC article.
-
Impact of maternal immune activation and sex on placental and fetal brain cytokine and gene expression profiles in a preclinical model of neurodevelopmental disorders.J Neuroinflammation. 2024 May 7;21(1):118. doi: 10.1186/s12974-024-03106-7. J Neuroinflammation. 2024. PMID: 38715090 Free PMC article.
Cited by
-
Developmental programming of tissue-resident macrophages.Front Immunol. 2024 Nov 7;15:1475369. doi: 10.3389/fimmu.2024.1475369. eCollection 2024. Front Immunol. 2024. PMID: 39575254 Free PMC article. Review.
-
The microbiome as a modulator of neurological health across the maternal-offspring interface.J Clin Invest. 2025 Feb 17;135(4):e184314. doi: 10.1172/JCI184314. J Clin Invest. 2025. PMID: 39959974 Free PMC article. Review.
-
Microglia as critical mediators linking perinatal immune stress to mental health trajectories.Neuropsychopharmacology. 2025 Jul 9. doi: 10.1038/s41386-025-02162-8. Online ahead of print. Neuropsychopharmacology. 2025. PMID: 40629104 Review.
-
The evidence of influenza A virus infection during pregnancy as a risk factor for neuropsychiatric disorder in offspring.Mol Psychiatry. 2025 Aug;30(8):3835-3836. doi: 10.1038/s41380-025-03059-0. Epub 2025 May 15. Mol Psychiatry. 2025. PMID: 40374760 No abstract available.
References
-
- Dawood FS, Kittikraisak W, Patel A, Rentz Hunt D, Suntarattiwong P, Wesley MG, et al. Incidence of influenza during pregnancy and association with pregnancy and perinatal outcomes in three middle-income countries: a multisite prospective longitudinal cohort study. Lancet Infect Dis. 2021;21:97–106. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

