WTAP/IGF2BP3-mediated GBE1 expression accelerates the proliferation and enhances stemness in pancreatic cancer cells via upregulating c-Myc
- PMID: 38961325
- PMCID: PMC11223412
- DOI: 10.1186/s11658-024-00611-8
WTAP/IGF2BP3-mediated GBE1 expression accelerates the proliferation and enhances stemness in pancreatic cancer cells via upregulating c-Myc
Abstract
Background: Pancreatic cancer (PC) is one of the most malignant cancers with highly aggressiveness and poor prognosis. N6-methyladenosine (m6A) have been indicated to be involved in PC development. Glucan Branching Enzyme 1 (GBE1) is mainly involved in cell glycogen metabolism. However, the function of GBE1 and Whether GBE1 occurs m6A modification in PC progression remains to be illustrated.
Methods: The clinical prognosis of GBE1 was analyzed through online platform. The expression of GBE1 was obtained from online platform and then verified in normal and PC cell lines. Lentivirus was used to generated GBE1 stable-overexpression or knockdown PC cells. Cell Counting Kit (CCK-8), colony formation assay, sphere formation assay and flow cytometry assay were conducted to analyze cell proliferation and stemness ability in vitro. Subcutaneous and orthotopic mouse models were used to verify the function of GBE1 in vivo. RNA immunoprecipitation (RIP) assay, RNA stability experiment and western blots were conducted to explore the molecular regulation of GBE1 in PC.
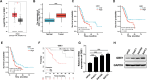
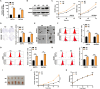
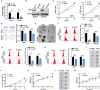
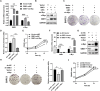
Results: GBE1 was significantly upregulated in PC and associated with poor prognosis of PC patients. Functionally, GBE1 overexpression facilitated PC cell proliferation and stemness-like properties, while knockdown of GBE1 attenuated the malignancy of PC cells. Importantly, we found the m6A modification of GBE1 RNA, and WTAP and IGF2BP3 was revealed as the m6A regulators to increase GBE1 mRNA stability and expression. Furthermore, c-Myc was discovered as a downstream gene of GBE1 and functional rescue experiments showed that overexpression of c-Myc could rescue GBE1 knockdown-induced PC cell growth inhibition.
Conclusions: Our study uncovered the oncogenic role of GBE1/c-Myc axis in PC progression and revealed WTAP/IGF2BP3-mediated m6A modification of GBE1, which highlight the potential application of GBE1 in the targeted therapy of PC.
Keywords: GBE1; IGF2BP3; Pancreatic cancer; Proliferation; WTAP; m6A modification.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
The Mechanism and Latest Progress of m6A Methylation in the Progression of Pancreatic Cancer.Int J Biol Sci. 2025 Jan 13;21(3):1187-1201. doi: 10.7150/ijbs.104407. eCollection 2025. Int J Biol Sci. 2025. PMID: 39897038 Free PMC article. Review.
-
WTAP-mediated N6-methyladenine Modification of circEEF2 Promotes Lung Adenocarcinoma Tumorigenesis by Stabilizing CANT1 in an IGF2BP2-dependent Manner.Mol Biotechnol. 2025 Apr;67(4):1494-1508. doi: 10.1007/s12033-024-01134-5. Epub 2024 Apr 15. Mol Biotechnol. 2025. PMID: 38619801
-
Hypoxia-Induced Up-Regulation of ACSS2 Drives the PI3K/AKT/mTOR Pathway Through HMGCS1 to Enhance the Proliferation and Stemness of Pancreatic Cancer Cells.Discov Med. 2025 Jun;37(197):1049-1061. doi: 10.24976/Discov.Med.202537197.93. Discov Med. 2025. PMID: 40485521
-
Gemcitabine Inhibits the Progression of Pancreatic Cancer by Restraining the WTAP/MYC Chain in an m6A-Dependent Manner.Cancer Res Treat. 2024 Jan;56(1):259-271. doi: 10.4143/crt.2022.1600. Epub 2023 Aug 16. Cancer Res Treat. 2024. PMID: 37591781 Free PMC article.
-
Stabilization of SQLE mRNA by WTAP/FTO/IGF2BP3-dependent manner in HGSOC: implications for metabolism, stemness, and progression.Cell Death Dis. 2024 Dec 1;15(12):872. doi: 10.1038/s41419-024-07257-6. Cell Death Dis. 2024. PMID: 39617776 Free PMC article.
Cited by
-
Biological roles of enhancer RNA m6A modification and its implications in cancer.Cell Commun Signal. 2025 May 30;23(1):254. doi: 10.1186/s12964-025-02254-4. Cell Commun Signal. 2025. PMID: 40448182 Free PMC article. Review.
-
The Mechanism and Latest Progress of m6A Methylation in the Progression of Pancreatic Cancer.Int J Biol Sci. 2025 Jan 13;21(3):1187-1201. doi: 10.7150/ijbs.104407. eCollection 2025. Int J Biol Sci. 2025. PMID: 39897038 Free PMC article. Review.
-
Identification of IGF2BP3 for the Expression and Prognosis in Gastrointestinal Cancers.Int J Med Sci. 2025 Jun 23;22(13):3191-3201. doi: 10.7150/ijms.114411. eCollection 2025. Int J Med Sci. 2025. PMID: 40765570 Free PMC article.
-
Role of the TGF-β/Smad3 pathway in pancreatic cancer cell growth and stem cell characteristics.Discov Oncol. 2025 Aug 6;16(1):1480. doi: 10.1007/s12672-025-03220-9. Discov Oncol. 2025. PMID: 40768128 Free PMC article.
-
The role of N(6)-methyladenosine (m6a) modification in cancer: recent advances and future directions.EXCLI J. 2025 Jan 15;24:113-150. doi: 10.17179/excli2024-7935. eCollection 2025. EXCLI J. 2025. PMID: 39967906 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- 82103295/National Natural Science Foundation of China
- 81801642/National Natural Science Foundation of China
- LQ22H160062/Natural Science Foundation of Zhejiang Province
- 2019RC105/Medical Science and Technology Project of Zhejiang Province
- 2022KY516/Medical Science and Technology Project of Zhejiang Province
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

