Early change of plasma Epstein-Barr virus DNA load and the viral lytic genome level could positively predict clinical outcome in recurrent or metastatic nasopharyngeal carcinoma receiving anti-programmed cell death 1 monotherapy
- PMID: 38961378
- PMCID: PMC11223362
- DOI: 10.1186/s12885-024-12564-4
Early change of plasma Epstein-Barr virus DNA load and the viral lytic genome level could positively predict clinical outcome in recurrent or metastatic nasopharyngeal carcinoma receiving anti-programmed cell death 1 monotherapy
Abstract
Purpose: Patients with recurrent or metastatic nasopharyngeal carcinoma (RM-NPC) have proven benefit from anti-programmed cell death 1 (anti-PD-1) monotherapy. Here, we retrospectively analyze the association of plasma Epstein-Barr virus (EBV) DNA load and tumor viral lytic genome with clinical outcome from 2 registered phase I trials.
Methods: Patients with RM-NPC from Checkmate 077 (nivolumab phase I trial in China) and Camrelizumab phase I trial between March 2016 and January 2018 were enrolled. Baseline EBV DNA titers were tested in 68 patients and EBV assessment was performed in 60 patients who had at least 3 post-baseline timepoints of EBV data and at least 1 post-baseline timepoint of radiographic assessment. We defined "EBV response" as 3 consecutive timepoints of load below 50% of baseline, and "EBV progression" as 3 consecutive timepoints of load above 150% of baseline. Whole-exome sequencing was performed in 60 patients with available tumor samples.
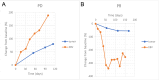
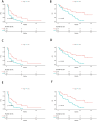
Results: We found that the baseline EBV DNA load was positively correlated with tumor size (spearman p < 0.001). Both partial response (PR) and stable disease (SD) patients had significantly lower EBV load than progression disease (PD) patients. EBV assessment was highly consistent with radiographic evaluation. Patients with EBV response had significantly improved overall survival (OS) than patients with EBV progression (log-rank p = 0.004, HR = 0.351 [95% CI: 0.171-0.720], median 22.5 vs. 11.9 months). The median time to initial EBV response and progression were 25 and 36 days prior to initial radiographic response and progression, respectively. Patients with high levels of EBV lytic genomes at baseline, including BKRF2, BKRF3 and BKRF4, had better progression-free survival (PFS) and OS.
Conclusion: In summary, early clearance of plasma EBV DNA load and high levels of lytic EBV genes were associated with better clinical outcome in patients with RM-NPC receiving anti-PD-1 monotherapy.
Keywords: Biomarkers; EBV; Immunotherapy; NPC.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Association of Plasma Epstein-Barr Virus DNA With Outcomes for Patients With Recurrent or Metastatic Nasopharyngeal Carcinoma Receiving Anti-Programmed Cell Death 1 Immunotherapy.JAMA Netw Open. 2022 Mar 1;5(3):e220587. doi: 10.1001/jamanetworkopen.2022.0587. JAMA Netw Open. 2022. PMID: 35230439 Free PMC article. Clinical Trial.
-
Efficacy, Safety, and Correlative Biomarkers of Toripalimab in Previously Treated Recurrent or Metastatic Nasopharyngeal Carcinoma: A Phase II Clinical Trial (POLARIS-02).J Clin Oncol. 2021 Mar 1;39(7):704-712. doi: 10.1200/JCO.20.02712. Epub 2021 Jan 25. J Clin Oncol. 2021. PMID: 33492986 Free PMC article. Clinical Trial.
-
Anti-epidermal growth factor receptor monoclonal antibody plus palliative chemotherapy as a first-line treatment for recurrent or metastatic nasopharyngeal carcinoma.Cancer Med. 2020 Mar;9(5):1721-1732. doi: 10.1002/cam4.2838. Epub 2020 Jan 19. Cancer Med. 2020. PMID: 31955525 Free PMC article. Clinical Trial.
-
Biomarkers predicting clinical outcomes in nasopharyngeal cancer patients receiving immune checkpoint inhibitors: A systematic review and meta-analysis.Front Immunol. 2023 Mar 31;14:1146898. doi: 10.3389/fimmu.2023.1146898. eCollection 2023. Front Immunol. 2023. PMID: 37063822 Free PMC article.
-
Prognostic value of Epstein-Barr virus DNA level for nasopharyngeal carcinoma: a meta-analysis of 8128 cases.Eur Arch Otorhinolaryngol. 2020 Jan;277(1):9-18. doi: 10.1007/s00405-019-05699-9. Epub 2019 Oct 28. Eur Arch Otorhinolaryngol. 2020. PMID: 31659449 Review.
Cited by
-
Viral oncogenesis in cancer: from mechanisms to therapeutics.Signal Transduct Target Ther. 2025 May 12;10(1):151. doi: 10.1038/s41392-025-02197-9. Signal Transduct Target Ther. 2025. PMID: 40350456 Free PMC article. Review.
-
Long-term immune checkpoint inhibitor therapy in a patient with metastatic nasopharyngeal carcinoma: a case report.Front Immunol. 2025 Jun 12;16:1585844. doi: 10.3389/fimmu.2025.1585844. eCollection 2025. Front Immunol. 2025. PMID: 40574835 Free PMC article.
-
Microbiome meets immunotherapy: unlocking the hidden predictors of immune checkpoint inhibitors.NPJ Biofilms Microbiomes. 2025 Sep 2;11(1):180. doi: 10.1038/s41522-025-00819-2. NPJ Biofilms Microbiomes. 2025. PMID: 40897718 Free PMC article.
-
Induction Camrelizumab and Modified TPF (Nab-Paclitaxel, Cisplatin, and S-1) in Locoregionally Advanced Nasopharyngeal Carcinoma.Cancer Sci. 2025 Jun;116(6):1616-1626. doi: 10.1111/cas.70037. Epub 2025 Mar 3. Cancer Sci. 2025. PMID: 40031812 Free PMC article.
References
-
- Ma BBY, Lim WT, Goh BC, Hui EP, Lo KW, Pettinger A, Foster NR, Riess JW, Agulnik M, Chang AYC, et al. Antitumor Activity of Nivolumab in recurrent and metastatic nasopharyngeal carcinoma: an International, Multicenter Study of the Mayo Clinic Phase 2 Consortium (NCI-9742) J Clin Oncol. 2018;36(14):1412–8. doi: 10.1200/JCO.2017.77.0388. - DOI - PMC - PubMed
-
- Fang W, Yang Y, Ma Y, Hong S, Lin L, He X, Xiong J, Li P, Zhao H, Huang Y, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol. 2018;19(10):1338–50. doi: 10.1016/S1470-2045(18)30495-9. - DOI - PubMed
-
- Wang FH, Wei XL, Feng J, Li Q, Xu N, Hu XC, Liao W, Jiang Y, Lin XY, Zhang QY, et al. Efficacy, Safety, and Correlative Biomarkers of Toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: a phase II clinical trial (POLARIS-02) J Clin Oncol. 2021;39(7):704–12. doi: 10.1200/JCO.20.02712. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

