Choroid plexus volume as a novel candidate neuroimaging marker of the Alzheimer's continuum
- PMID: 38961406
- PMCID: PMC11221040
- DOI: 10.1186/s13195-024-01520-w
Choroid plexus volume as a novel candidate neuroimaging marker of the Alzheimer's continuum
Abstract
Background: Enlarged choroid plexus (ChP) volume has been reported in patients with Alzheimer's disease (AD) and inversely correlated with cognitive performance. However, its clinical diagnostic and predictive value, and mechanisms by which ChP impacts the AD continuum remain unclear.
Methods: This prospective cohort study enrolled 607 participants [healthy control (HC): 110, mild cognitive impairment (MCI): 269, AD dementia: 228] from the Chinese Imaging, Biomarkers, and Lifestyle study between January 1, 2021, and December 31, 2022. Of the 497 patients on the AD continuum, 138 underwent lumbar puncture for cerebrospinal fluid (CSF) hallmark testing. The relationships between ChP volume and CSF pathological hallmarks (Aβ42, Aβ40, Aβ42/40, tTau, and pTau181), neuropsychological tests [Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), Neuropsychiatric Inventory (NPI), and Activities of Daily Living (ADL) scores], and multimodal neuroimaging measures [gray matter volume, cortical thickness, and corrected cerebral blood flow (cCBF)] were analyzed using partial Spearman's correlation. The mediating effects of four neuroimaging measures [ChP volume, hippocampal volume, lateral ventricular volume (LVV), and entorhinal cortical thickness (ECT)] on the relationship between CSF hallmarks and neuropsychological tests were examined. The ability of the four neuroimaging measures to identify cerebral Aβ42 changes or differentiate among patients with AD dementia, MCI and HCs was determined using receiver operating characteristic analysis, and their associations with neuropsychological test scores at baseline were evaluated by linear regression. Longitudinal associations between the rate of change in the four neuroimaging measures and neuropsychological tests scores were evaluated on the AD continuum using generalized linear mixed-effects models.
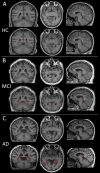
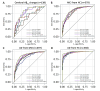
Results: The participants' mean age was 65.99 ± 8.79 years. Patients with AD dementia exhibited the largest baseline ChP volume than the other groups (P < 0.05). ChP volume enlargement correlated with decreased Aβ42 and Aβ40 levels; lower MMSE and MoCA and higher NPI and ADL scores; and lower volume, cortical thickness, and cCBF in other cognition-related regions (all P < 0.05). ChP volume mediated the association of Aβ42 and Aβ40 levels with MMSE scores (19.08% and 36.57%), and Aβ42 levels mediated the association of ChP volume and MMSE or MoCA scores (39.49% and 34.36%). ChP volume alone better identified cerebral Aβ42 changes than LVV alone (AUC = 0.81 vs. 0.67, P = 0.04) and EC thickness alone (AUC = 0.81 vs.0.63, P = 0.01) and better differentiated patients with MCI from HCs than hippocampal volume alone (AUC = 0.85 vs. 0.81, P = 0.01), and LVV alone (AUC = 0.85 vs.0.82, P = 0.03). Combined ChP and hippocampal volumes significantly increased the ability to differentiate cerebral Aβ42 changes and patients among AD dementia, MCI, and HCs groups compared with hippocampal volume alone (all P < 0.05). After correcting for age, sex, years of education, APOE ε4 status, eTIV, and hippocampal volume, ChP volume was associated with MMSE, MoCA, NPI, and ADL score at baseline, and rapid ChP volume enlargement was associated with faster deterioration in NPI scores with an average follow-up of 10.03 ± 4.45 months (all P < 0.05).
Conclusions: ChP volume may be a novel neuroimaging marker associated with neurodegenerative changes and clinical AD manifestations. It could better detect the early stages of the AD and predict prognosis, and significantly enhance the differential diagnostic ability of hippocampus on the AD continuum.
Keywords: Alzheimer’s disease; Amyloid-beta; Choroid plexus; Longitudinal studies; Mediation analysis; ROC curve.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

