The impact of regional disparities on the availability of meningococcal vaccines in the US
- PMID: 38961431
- PMCID: PMC11221024
- DOI: 10.1186/s12889-024-19081-w
The impact of regional disparities on the availability of meningococcal vaccines in the US
Abstract
Background: In the United States (US), three types of vaccines are available to prevent invasive meningococcal disease (IMD), a severe and potentially fatal infection: quadrivalent conjugate vaccines against serogroups A, C, W, Y (MenACWY), and monovalent vaccines against serogroup B (MenB) as well as a newly licensed pentavalent vaccine (MenABCWY) protecting against serogroup A, B, C, W, and Y. The CDC's Advisory Committee on Immunization Practices (ACIP) routinely recommends MenACWY vaccine for all 11- to 12-year-olds with a booster dose at 16 years. MenB vaccination is recommended based on shared clinical decision-making (SCDM) for 16- to 23-year-olds. Recently, the pentavalent meningococcal vaccine (MenABCWY) was recommended by the ACIP. Meningococcal vaccine uptake is suboptimal across the country, particularly among individuals with lower socioeconomic status (SES), despite these recommendations. The objective of the spatial analyses was to assess the relationship between stocking of MenACWY and MenB vaccines, area-level SES, and state-level policies.
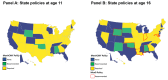
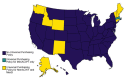
Methods: The number of MenACWY and MenB doses stocked by vaccinators was obtained from IQVIA and the CDC's Vaccine for Children (VFC) program and compiled into a county-level dataset from 2016 to 2019. SES, as measured using the CDC's Social Vulnerability Index (SVI), state-level school recommendations, and universal purchasing programs were among the main county-level covariates included to control for factors likely influencing stocking. Data were stratified by public and private market. Bayesian spatial regression models were developed to quantify the variations in rates of stocking and the relative rates of stocking of both vaccines.
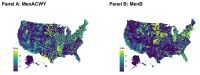
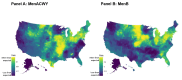
Results: After accounting for county-level characteristics, lower SES counties tended to have fewer doses of MenB relative to MenACWY on both public and private markets. Lower SES counties tended to have more supply of public vs. private doses. Universal purchasing programs had a strong effect on the markets for both vaccines shifting nearly all doses to the public market. School vaccination strategy was key for improving stocking rates.
Conclusions: Overall, the results show that MenACWY has greater stock relative to MenB across the US. This difference is exacerbated in vulnerable areas without school entry requirements for vaccination and results in inequity of vaccine availability. Beyond state-level policy and SES differences, SCDM recommendations may be a contributing factor, although this was not directly assessed by our model.
Keywords: Access barriers; Inequality; Invasive meningococcal disease; Meningococcal vaccination; Shared clinical decision making; Social equity; Vaccine access.
© 2024. The Author(s).
Conflict of interest statement
KS, EJ, JVP, SJW, CGP are employees of Pfizer and may hold stocks or stock options of Pfizer Inc.
Figures
Similar articles
-
Inequalities in the risk and prevention of invasive meningococcal disease in the United States - A systematic literature review.Hum Vaccin Immunother. 2024 Dec 31;20(1):2406613. doi: 10.1080/21645515.2024.2406613. Epub 2024 Oct 7. Hum Vaccin Immunother. 2024. PMID: 39373020 Free PMC article.
-
Immunogenicity and safety of a pentavalent meningococcal ABCWY vaccine in adolescents and young adults: an observer-blind, active-controlled, randomised trial.Lancet Infect Dis. 2023 Dec;23(12):1370-1382. doi: 10.1016/S1473-3099(23)00191-3. Epub 2023 Aug 11. Lancet Infect Dis. 2023. PMID: 37579773 Clinical Trial.
-
Potential public health impact of a Neisseria meningitidis A, B, C, W, and Y pentavalent vaccine in the United States.Postgrad Med. 2022 May;134(4):341-348. doi: 10.1080/00325481.2021.1876478. Epub 2021 Feb 22. Postgrad Med. 2022. PMID: 33615973
-
Meningococcal Vaccination: Recommendations of the Advisory Committee on Immunization Practices, United States, 2020.MMWR Recomm Rep. 2020 Sep 25;69(9):1-41. doi: 10.15585/mmwr.rr6909a1. MMWR Recomm Rep. 2020. PMID: 33417592 Free PMC article.
-
Meningococcal Vaccination of Adolescents in the United States: Past Successes and Future Considerations.J Adolesc Health. 2024 Jun;74(6):1068-1077. doi: 10.1016/j.jadohealth.2024.01.016. Epub 2024 Mar 2. J Adolesc Health. 2024. PMID: 38430074 Review.
Cited by
-
Understanding the value of meningococcal vaccination for adolescents and young adults in the United States: insights from a steady-state modelling approach.BMC Public Health. 2025 May 20;25(1):1863. doi: 10.1186/s12889-025-21953-8. BMC Public Health. 2025. PMID: 40394570 Free PMC article.
-
Inequalities in the risk and prevention of invasive meningococcal disease in the United States - A systematic literature review.Hum Vaccin Immunother. 2024 Dec 31;20(1):2406613. doi: 10.1080/21645515.2024.2406613. Epub 2024 Oct 7. Hum Vaccin Immunother. 2024. PMID: 39373020 Free PMC article.
-
Quadrivalent Conjugate Vaccine and Invasive Meningococcal Disease in US Adolescents and Young Adults.JAMA Netw Open. 2024 Nov 4;7(11):e2443551. doi: 10.1001/jamanetworkopen.2024.43551. JAMA Netw Open. 2024. PMID: 39504021 Free PMC article.
References
-
- CDC. Enhanced Meningococcal Disease Surveillance Report. 2019. Accessed October 5, 2023. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2019.pdf
-
- CDC. Enhanced Meningococcal Disease Surveillance Report. 2021. Date: June 21, 2023. Accessed: October 10, 2023. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2021.pdf
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

