Plasma biomarkers of amyloid, tau, axonal, and neuroinflammation pathologies in dementia with Lewy bodies
- PMID: 38961441
- PMCID: PMC11221164
- DOI: 10.1186/s13195-024-01502-y
Plasma biomarkers of amyloid, tau, axonal, and neuroinflammation pathologies in dementia with Lewy bodies
Abstract
Background: Increasing evidence supports the use of plasma biomarkers of amyloid, tau, neurodegeneration, and neuroinflammation for diagnosis of dementia. However, their performance for positive and differential diagnosis of dementia with Lewy bodies (DLB) in clinical settings is still uncertain.
Methods: We conducted a retrospective biomarker study in two tertiary memory centers, Paris Lariboisière and CM2RR Strasbourg, France, enrolling patients with DLB (n = 104), Alzheimer's disease (AD, n = 76), and neurological controls (NC, n = 27). Measured biomarkers included plasma Aβ40/Aβ42 ratio, p-tau181, NfL, and GFAP using SIMOA and plasma YKL-40 and sTREM2 using ELISA. DLB patients with available CSF analysis (n = 90) were stratified according to their CSF Aβ profile.
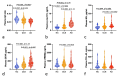
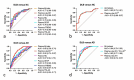
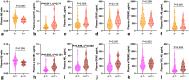
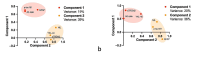
Results: DLB patients displayed modified plasma Aβ ratio, p-tau181, and GFAP levels compared with NC and modified plasma Aβ ratio, p-tau181, GFAP, NfL, and sTREM2 levels compared with AD patients. Plasma p-tau181 best differentiated DLB from AD patients (ROC analysis, area under the curve [AUC] = 0.80) and NC (AUC = 0.78), and combining biomarkers did not improve diagnosis performance. Plasma p-tau181 was the best standalone biomarker to differentiate amyloid-positive from amyloid-negative DLB cases (AUC = 0.75) and was associated with cognitive status in the DLB group. Combining plasma Aβ ratio, p-tau181 and NfL increased performance to identify amyloid copathology (AUC = 0.79). Principal component analysis identified different segregation patterns of biomarkers in the DLB and AD groups.
Conclusions: Amyloid, tau, neurodegeneration and neuroinflammation plasma biomarkers are modified in DLB, albeit with moderate diagnosis performance. Plasma p-tau181 can contribute to identify Aβ copathology in DLB.
Keywords: Alzheimer’s disease; Amyloid pathology; Dementia with Lewy bodies; Plasma biomarkers.
© 2024. The Author(s).
Conflict of interest statement
F.B. was the national coordinator for France for the Eisai Delphia (E2027), Axovant Headway-DLB and Roche Graduate therapeutic trials; he had received honoraria from Roche, Eisai and Biogen for oral presentations, and from Eisai for a board. C.P. is a member of the International Advisory Boards of Lilly; is a consultant for Fujiribio, Alzhois, Neuroimmune, Ads Neuroscience, Roche, AgenT and Gilead; and is involved as an investigator in several clinical trials for Roche, Esai, Lilly, Biogen, Astrazeneca, Lundbeck, and Neuroimmune. All other authors report no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

