The efficacy of simple oral nutritional supplements versus usual care in postoperative patients with gastric cancer: study protocol for a multicenter, open-label, parallel, randomized controlled trial
- PMID: 38961505
- PMCID: PMC11223300
- DOI: 10.1186/s13063-024-08169-8
The efficacy of simple oral nutritional supplements versus usual care in postoperative patients with gastric cancer: study protocol for a multicenter, open-label, parallel, randomized controlled trial
Abstract
Background: Body weight loss (BWL) after gastrectomy impact on the short- and long-term outcomes. Oral nutritional supplement (ONS) has potential to prevent BWL in patients after gastrectomy. However, there is no consistent evidence supporting the beneficial effects of ONS on BWL, muscle strength and health-related quality of life (HRQoL). This study aimed to evaluate the effects of ONS formulated primarily with carbohydrate and protein on BWL, muscle strength, and HRQoL.
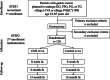
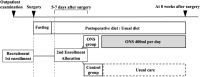
Methods: This will be a multicenter, open-label, parallel, randomized controlled trial in patients with gastric cancer who will undergo gastrectomy. A total of 120 patients who will undergo gastrectomy will be randomly assigned to the ONS group or usual care (control) group in a 1:1 ratio. The stratification factors will be the clinical stage (I or ≥ II) and surgical procedures (total gastrectomy or other procedure). In the ONS group, the patients will receive 400 kcal (400 ml)/day of ONS from postoperative day 5 to 7, and the intervention will continue postoperatively for 8 weeks. The control group patients will be given a regular diet. The primary outcome will be the percentage of BWL (%BWL) from baseline to 8 weeks postoperatively. The secondary outcomes will be muscle strength (handgrip strength), HRQoL (EORTC QLQ-C30, QLQ-OG25, EQ-5D-5L), nutritional status (hemoglobin, lymphocyte count, albumin), and dietary intake. All analyses will be performed on an intention-to-treat basis.
Discussion: This study will provide evidence showing whether or not ONS with simple nutritional ingredients can improve patient adherence and HRQoL by reducing BWL after gastrectomy. If supported by the study results, nutritional support with simple nutrients will be recommended to patients after gastrectomy for gastric cancer.
Trial registration: jRCTs051230012; Japan Registry of Clinical Trails. Registered on Apr. 13, 2023.
Keywords: Body weight loss; Gastrectomy; Gastric cancer; Oral nutritional supplement; Patient-reported outcomes; Quality of life.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Effect of Oral Nutritional Supplements Composed of High Protein on Body Weight Loss After Gastrectomy.In Vivo. 2025 Jan-Feb;39(1):426-432. doi: 10.21873/invivo.13845. In Vivo. 2025. PMID: 39740898 Free PMC article.
-
Efficacy of Simple Oral Nutritional Supplements versus Usual Care in Postoperative Patients with Gastric Cancer: A Multicenter, Open-label, Parallel, Randomized Controlled Trial.Ann Surg. 2025 Aug 7. doi: 10.1097/SLA.0000000000006888. Online ahead of print. Ann Surg. 2025. PMID: 40772645
-
Oral nutritional supplements versus a regular diet alone for body weight loss after gastrectomy: a phase 3, multicenter, open-label randomized controlled trial.Gastric Cancer. 2021 Sep;24(5):1150-1159. doi: 10.1007/s10120-021-01188-3. Epub 2021 Apr 9. Gastric Cancer. 2021. PMID: 33835329 Clinical Trial.
-
Effects of nutritional interventions on nutritional status in patients with gastric cancer: A systematic review and meta-analysis of randomized controlled trials.Clin Nutr ESPEN. 2020 Aug;38:28-42. doi: 10.1016/j.clnesp.2020.05.007. Epub 2020 Jun 16. Clin Nutr ESPEN. 2020. PMID: 32690170
-
Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: current evidence and guidance for design of future trials.Ann Oncol. 2018 May 1;29(5):1141-1153. doi: 10.1093/annonc/mdy114. Ann Oncol. 2018. PMID: 29788170 Free PMC article.
References
-
- Tawantanakorn T, Phibalyart W, Parakonthun T, et al. Changes in physical components after Gastrectomy for adenocarcinoma of stomach and esophagogastric junction. Siriraj Med J. 2023;75(4):241–249. doi: 10.33192/smj.v75i4.260962. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous