Interface-Mediated Neurogenic Signaling: The Impact of Surface Geometry and Chemistry on Neural Cell Behavior for Regenerative and Brain-Machine Interfacing Applications
- PMID: 38961531
- PMCID: PMC11326983
- DOI: 10.1002/adma.202401750
Interface-Mediated Neurogenic Signaling: The Impact of Surface Geometry and Chemistry on Neural Cell Behavior for Regenerative and Brain-Machine Interfacing Applications
Abstract
Nanomaterial advancements have driven progress in central and peripheral nervous system applications such as tissue regeneration and brain-machine interfacing. Ideally, neural interfaces with native tissue shall seamlessly integrate, a process that is often mediated by the interfacial material properties. Surface topography and material chemistry are significant extracellular stimuli that can influence neural cell behavior to facilitate tissue integration and augment therapeutic outcomes. This review characterizes topographical modifications, including micropillars, microchannels, surface roughness, and porosity, implemented on regenerative scaffolding and brain-machine interfaces. Their impact on neural cell response is summarized through neurogenic outcome and mechanistic analysis. The effects of surface chemistry on neural cell signaling with common interfacing compounds like carbon-based nanomaterials, conductive polymers, and biologically inspired matrices are also reviewed. Finally, the impact of these extracellular mediated neural cues on intracellular signaling cascades is discussed to provide perspective on the manipulation of neuron and neuroglia cell microenvironments to drive therapeutic outcomes.
Keywords: cell signaling; neural Interfaces; neurogenesis; substrate chemistry; surface topographies.
© 2024 The Author(s). Advanced Materials published by Wiley‐VCH GmbH.
Figures




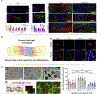


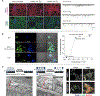


Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Computational Fluid Dynamics Modeling of Material Transport Through Triply Periodic Minimal Surface Scaffolds for Bone Tissue Engineering.J Biomech Eng. 2025 Mar 1;147(3):031007. doi: 10.1115/1.4067575. J Biomech Eng. 2025. PMID: 39790065
-
In mice, discrete odors can selectively promote the neurogenesis of sensory neuron subtypes that they stimulate.Elife. 2025 Jun 18;13:RP96152. doi: 10.7554/eLife.96152. Elife. 2025. PMID: 40531183 Free PMC article.
-
Property-tailoring chemical modifications of hyaluronic acid for regenerative medicine applications.Acta Biomater. 2025 Jul 1;201:75-100. doi: 10.1016/j.actbio.2025.06.014. Epub 2025 Jun 7. Acta Biomater. 2025. PMID: 40490241 Review.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
Cited by
-
Biomaterials for neuroengineering: applications and challenges.Regen Biomater. 2025 Feb 21;12:rbae137. doi: 10.1093/rb/rbae137. eCollection 2025. Regen Biomater. 2025. PMID: 40007617 Free PMC article. Review.
References
-
- Strumwasser F, “Long-term recording from single neurons in brain of unrestrained mammals,” Science, vol. 127, no. 3296, pp. 469–470, 1958. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

