Conjugated hyperbilirubinemia associated with accidental rifapentine overdose
- PMID: 38961547
- PMCID: PMC11392536
- DOI: 10.5588/ijtld.23.0494
Conjugated hyperbilirubinemia associated with accidental rifapentine overdose
Conflict of interest statement
Conflicts of interest: JM, DH, ES, KS, IW, and GM have institutional funding from NIH. JM is also chair of the NIH ACTG Trial A5362. ES has institutional funding from UNITE4TB, a Veni Grant from the Dutch Research Council (The Hague, Utrecht, The Netherlands), and a UNITAID grant. ES has research collaborations with TB Alliance (New York, NY, USA) and Janssen Pharmaceuticals (Beerse, Belgium). ES is a DSMB member for the BE-PEOPLE Study and a member of the CHEETA Task Force. The remaining authors do not have any conflicts of interest.
Figures
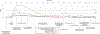
References
-
- Metcalfe J. Clofazimine- and rifapentine-containing treatment shortening regimens in drug-susceptible tuberculosis: The CLO-FAST Study. ClinicalTrials.gov, 2020. NCT04311502. https://clinicaltrials.gov/ct2/show/NCT04311502 Accessed December 2022.
-
- Frick M. An activist’s guide to rifapentine for the treatment of TB infection. Treatment Action Group, 2020. https://www.treatmentactiongroup.org/publication/an-activists-guide-to-r... Accessed January 2023.
-
- Chung JY, et al. Effect of OATP1B1 (SLCO1B1) variant alleles on the pharmacokinetics of pitavastatin in healthy volunteers. Clin Pharmacol Ther 2005;78(4):342–350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

