Protecting TiS3 Photoanodes for Water Splitting in Alkaline Media by TiO2 Coatings
- PMID: 38961573
- PMCID: PMC11231970
- DOI: 10.1021/acsami.4c07404
Protecting TiS3 Photoanodes for Water Splitting in Alkaline Media by TiO2 Coatings
Abstract
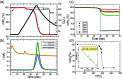
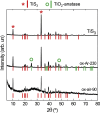
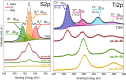
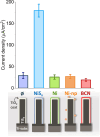
Titanium trisulfide (TiS3) nanoribbons, when coated with titanium dioxide (TiO2), can be used for water splitting in the KOH electrolyte. TiO2 shells can be prepared through thermal annealing to regulate the response of TiS3/TiO2 heterostructures by controlling the oxidation time and growth atmosphere. The thickness and structure of the TiO2 layers significantly influence the photoelectrocatalytic properties of the TiS3/TiO2 photoanodes, with amorphous layers showing better performance than crystalline ones. The oxide layers should be thin enough to transfer photogenerated charge through the electrode-electrolyte interface while protecting TiS3 from KOH corrosion. Finally, the performance of TiS3/TiO2 heterostructures has been improved by coating them with various electrocatalysts, NiSx being the most effective. This research presents new opportunities to create efficient semiconductor heterostructures to be used as photoanodes in corrosive alkaline aqueous solutions.
Keywords: KOH corrosion; TiO2 coatings; heterostructures; hydrogen production; photoelectrolysis; water-splitting.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
Borocarbonitride Layers on Titanium Dioxide Nanoribbons for Efficient Photoelectrocatalytic Water Splitting.Materials (Basel). 2021 Sep 23;14(19):5490. doi: 10.3390/ma14195490. Materials (Basel). 2021. PMID: 34639887 Free PMC article.
-
The influence of structure and processing on the behavior of TiO2 protective layers for stabilization of n-Si/TiO2/Ni photoanodes for water oxidation.ACS Appl Mater Interfaces. 2015 Jul 22;7(28):15189-99. doi: 10.1021/acsami.5b00379. Epub 2015 Jul 7. ACS Appl Mater Interfaces. 2015. PMID: 26083827
-
Tunable Photodetectors via In Situ Thermal Conversion of TiS3 to TiO2.Nanomaterials (Basel). 2020 Apr 9;10(4):711. doi: 10.3390/nano10040711. Nanomaterials (Basel). 2020. PMID: 32283697 Free PMC article.
-
Photoanodes based on TiO2 and α-Fe2O3 for solar water splitting - superior role of 1D nanoarchitectures and of combined heterostructures.Chem Soc Rev. 2017 Jun 19;46(12):3716-3769. doi: 10.1039/c6cs00015k. Chem Soc Rev. 2017. PMID: 28397882 Review.
-
Recent Advances in TiO2 -based Photoanodes for Photoelectrochemical Water Splitting.Chem Asian J. 2022 Oct 17;17(20):e202200668. doi: 10.1002/asia.202200668. Epub 2022 Sep 8. Chem Asian J. 2022. PMID: 35925726 Review.
References
-
- Osterloh F. E.; Parkinson B. A. Recent developments in solar water-splitting photocatalysis. MRS Bull. 2011, 36 (1), 17–22. 10.1557/mrs.2010.5. - DOI
LinkOut - more resources
Full Text Sources

