Transcriptomic and metabolomic changes might predict frailty in SAMP8 mice
- PMID: 38961613
- PMCID: PMC11464142
- DOI: 10.1111/acel.14263
Transcriptomic and metabolomic changes might predict frailty in SAMP8 mice
Abstract
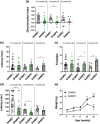
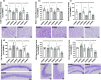
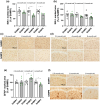
Frailty is a geriatric, multi-dimensional syndrome that reflects multisystem physiological change and is a transversal measure of reduced resilience to negative events. It is characterized by weakness, frequent falls, cognitive decline, increased hospitalization and dead and represents a risk factor for the development of Alzheimer's disease (AD). The fact that frailty is recognized as a reversible condition encourages the identification of earlier biomarkers to timely predict and prevent its occurrence. SAMP8 (Senescence-Accelerated Mouse Prone-8) mice represent the most appropriate preclinical model to this aim and were used in this study to carry transcriptional and metabolic analyses in the brain and plasma, respectively, upon a characterization at cognitive, motor, structural, and neuropathological level at 2.5, 6, and 9 months of age. At 2.5 months, SAMP8 mice started displaying memory deficits, muscle weakness, and motor impairment. Functional alterations were associated with a neurodevelopmental deficiency associated with reduced neuronal density and glial cell loss. Through transcriptomics, we identified specific genetic signatures well distinguishing SAMP8 mice at 6 months, whereas plasma metabolomics allowed to segregate SAMP8 mice from SAMR1 already at 2.5 months of age by detecting constitutively lower levels of acylcarnitines and lipids in SAMP8 at all ages investigated correlating with functional deficits and neuropathological signs. Our findings suggest that specific genetic alterations at central level, as well as metabolomic changes in plasma, might allow to early assess a frail condition leading to dementia development, which paves the foundation for future investigation in a clinical setting.
Keywords: aging; dementia; frailty; motor impairment; neuronal loss; predictive biomarkers; senescent SAMP8 mice.
© 2024 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
We have no conflicts of interest to declare.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

