Oral histidine affects gut microbiota and MAIT cells improving glycemic control in type 2 diabetes patients
- PMID: 38961712
- PMCID: PMC11225920
- DOI: 10.1080/19490976.2024.2370616
Oral histidine affects gut microbiota and MAIT cells improving glycemic control in type 2 diabetes patients
Abstract
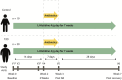
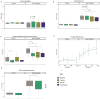
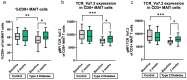
Amino acids, metabolized by host cells as well as commensal gut bacteria, have signaling effects on host metabolism. Oral supplementation of the essential amino acid histidine has been shown to exert metabolic benefits. To investigate whether dietary histidine aids glycemic control, we performed a case-controlled parallel clinical intervention study in participants with type 2 diabetes (T2D) and healthy controls. Participants received oral histidine for seven weeks. After 2 weeks of histidine supplementation, the microbiome was depleted by antibiotics to determine the microbial contribution to histidine metabolism. We assessed glycemic control, immunophenotyping of peripheral blood mononucelar cells (PBMC), DNA methylation of PBMCs and fecal gut microbiota composition. Histidine improves several markers of glycemic control, including postprandial glucose levels with a concordant increase in the proportion of MAIT cells after two weeks of histidine supplementation. The increase in MAIT cells was associated with changes in gut microbial pathways such as riboflavin biosynthesis and epigenetic changes in the amino acid transporter SLC7A5. Associations between the microbiome and MAIT cells were replicated in the MetaCardis cohort. We propose a conceptual framework for how oral histidine may affect MAIT cells via altered gut microbiota composition and SLC7A5 expression in MAIT cells directly and thereby influencing glycemic control. Future studies should focus on the role of flavin biosynthesis intermediates and SLC7A5 modulation in MAIT cells to modulate glycemic control.
Keywords: Microbiome; diabetes; histidine; insulin resistance; monocytes.
Conflict of interest statement
M Nieuwdorp is a member of the scientific advisory board of Caelus Health and all honoraria are paid to the employer Amsterdam University Medical Centres. F Backhed is a member of the scientific advisory board of Implexion. Karine Clement is a consultant for Danone Research, LNC Therapeutics and CONFO Therapeutics for work that is unassociated with the present study. Karine Clement has held a collaborative research contract with Danone Research in the context of the MetaCardis project. All honoraria are paid to the employer Sorbonne university. D van Raalte has participated in advisory boards for AstraZeneca, Boehringer Ingelheim‐Eli Lilly Alliance, MSD, Novo Nordisk and Sanofi, and has received research grants from AstraZeneca, Boehringer Ingelheim‐Eli Lilly Alliance, MSD and Sanofi. All honoraria are paid to the employer Amsterdam University Medical Centres. All other authors declare to have no related conflict of interest.
Figures


References
-
- Magliano DJ, Boyko EJ.. Idf diabetes atlas. 10th ed. Brussels: IDF Diabetes Atlas; 2021.
-
- Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, Rosas SE, Del Prato S, Mathieu C, Mingrone G, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753–20. doi:10.2337/dci22-0034. - DOI - PMC - PubMed
-
- ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, et al. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: standards of care in diabetes—2023. Diabetes Care. 2023;46(Supplement_1):S128–S139. doi:10.2337/dc23-S008. - DOI - PMC - PubMed
-
- Hooton D, Lentle R, Monro J, Wickham M, Simpson R. The secretion and action of brush border enzymes in the mammalian small intestine. Rev Physiol Biochem Pharmacol. 2015;168:59–118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
