Review of Pseudoloma neurophilia (Microsporidia): A common neural parasite of laboratory zebrafish (Danio rerio)
- PMID: 38961716
- PMCID: PMC11846143
- DOI: 10.1111/jeu.13040
Review of Pseudoloma neurophilia (Microsporidia): A common neural parasite of laboratory zebrafish (Danio rerio)
Abstract
Zebrafish (Danio rerio) is now the second most used animal model in biomedical research. As with other vertebrate models, underlying diseases and infections often impact research. Beyond mortality and morbidity, these conditions can compromise research end points by producing nonprotocol induced variation within experiments. Pseudoloma neurophilia, a microsporidium that targets the central nervous system, is the most frequently diagnosed pathogen in zebrafish facilities. The parasite undergoes direct, horizontal transmission within populations, and is also maternally transmitted with spores in ovarian fluid and occasionally within eggs. This transmission explains the wide distribution among research laboratories as new lines are generally introduced as embryos. The infection is chronic, and fish apparently never recover following the initial infection. However, most fish do not exhibit outward clinical signs. Histologically, the parasite occurs as aggregates of spores throughout the midbrain and spinal cord and extends to nerve roots. It often elicits meninxitis, myositis, and myodegeneration when it infects the muscle. There are currently no described therapies for the parasite, thus the infection is best avoided by screening with PCR-based tests and removal of infected fish from a facility. Examples of research impacts include reduced fecundity, behavioral changes, transcriptome alterations, and autofluorescent lesions.
Keywords: diagnostics; fish; infection; parasite; pathogenesis; transmission.
© 2024 International Society of Protistologists.
Figures



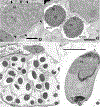
Similar articles
-
Verification of intraovum transmission of a microsporidium of vertebrates: Pseudoloma neurophilia infecting the Zebrafish, Danio rerio.PLoS One. 2013 Sep 23;8(9):e76064. doi: 10.1371/journal.pone.0076064. eCollection 2013. PLoS One. 2013. PMID: 24086686 Free PMC article.
-
Transmission of Pseudoloma neurophilia in Laboratory Zebrafish (Danio rerio) When Using Mass Spawning Chambers and Recommendations for Chamber Disinfection.Zebrafish. 2018 Feb;15(1):63-72. doi: 10.1089/zeb.2017.1493. Epub 2017 Oct 19. Zebrafish. 2018. PMID: 29048998 Free PMC article.
-
Polymerase chain reaction detection of Pseudoloma neurophilia, a common microsporidian of zebrafish (Danio rerio) reared in research laboratories.J Am Assoc Lab Anim Sci. 2006 Jan;45(1):36-9. J Am Assoc Lab Anim Sci. 2006. PMID: 16539333 Free PMC article.
-
Transmission, diagnosis, and recommendations for control of Pseudoloma neurophilia infections in laboratory zebrafish (Danio rerio) facilities.Comp Med. 2011 Aug;61(4):322-9. Comp Med. 2011. PMID: 22330247 Free PMC article. Review.
-
Documented and potential research impacts of subclinical diseases in zebrafish.ILAR J. 2012;53(2):126-34. doi: 10.1093/ilar.53.2.126. ILAR J. 2012. PMID: 23382344 Free PMC article. Review.
Cited by
-
Cryo-ET reveals the in situ architecture of the polar tube invasion apparatus from microsporidian parasites.Proc Natl Acad Sci U S A. 2025 Mar 18;122(11):e2415233122. doi: 10.1073/pnas.2415233122. Epub 2025 Mar 11. Proc Natl Acad Sci U S A. 2025. PMID: 40067903
References
-
- Caballero-Huertas M, Soto M, & Ribas L 2021. Reviewing Pseudoloma neurophilia infections in the popular zebrafish model. Rev. Aquacult, 13:1816–1827.
-
- Cali A, Kent M, Sanders J, Pau C, & Takvorian PM 2012. Development, ultrastructural pathology, and taxonomic revision of the microsporidial genus, Pseudoloma and its type species Pseudoloma neurophilia, in skeletal muscle and nervous tissue of experimentally infected zebrafish Danio rerio. J, Eukaryot. Microbiol, 59:40–48. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

