Self-reported neurocognitive complaints in the Swiss HIV Cohort Study: a viral genome-wide association study
- PMID: 38961872
- PMCID: PMC11220509
- DOI: 10.1093/braincomms/fcae188
Self-reported neurocognitive complaints in the Swiss HIV Cohort Study: a viral genome-wide association study
Abstract
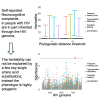
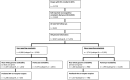
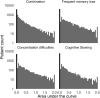
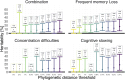
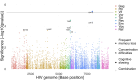
People with HIV may report neurocognitive complaints, with or without associated neurocognitive impairment, varying between individuals and populations. While the HIV genome could play a major role, large systematic viral genome-wide screens to date are lacking. The Swiss HIV Cohort Study biannually enquires neurocognitive complaints. We quantified broad-sense heritability estimates using partial 'pol' sequences from the Swiss HIV Cohort Study resistance database and performed a viral near full-length genome-wide association study for the longitudinal area under the curve of neurocognitive complaints. We performed all analysis (i) restricted to HIV Subtype B and (ii) including all HIV subtypes. From 8547 people with HIV with neurocognitive complaints, we obtained 6966 partial 'pol' sequences and 2334 near full-length HIV sequences. Broad-sense heritability estimates for presence of memory loss complaints ranged between 1% and 17% (Subtype B restricted 1-22%) and increased with the stringency of the phylogenetic distance thresholds. The genome-wide association study revealed one amino acid (Env L641E), after adjusting for multiple testing, positively associated with memory loss complaints (P = 4.3 * 10-6). Other identified mutations, while insignificant after adjusting for multiple testing, were reported in other smaller studies (Tat T64N, Env *291S). We present the first HIV genome-wide association study analysis of neurocognitive complaints and report a first estimate for the heritability of neurocognitive complaints through HIV. Moreover, we could identify one mutation significantly associated with the presence of memory loss complaints. Our findings indicate that neurocognitive complaints are polygenetic and highlight advantages of a whole genome approach for pathogenicity determination.
Keywords: HIV; cohort study; genome-wide association study; neurocognitive complaints; viral genome.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
An.R. received research grants from Gilead, paid to his institution; travel expenses from Gilead and Pfizer, paid to his institution; and honoraria for data safety monitoring board or advisory board consultations from MSD and Moderna, paid to his institution. C.P. received personal research grants from the Swiss HIV Cohort Study, Collegium Helveticum and the University of Zurich. E.B. received research grants from MSD, paid to his institution; consulting fees from Moderna, paid to his institution; honoraria for presentations from Pfizer, paid to his institution; travel expenses from ViiV, MSD, Gilead and Pfizer, paid to his institution; and honoraria for data safety monitoring board or advisory board consultations from ViiV, MSD, Pfizer, Gilead, Moderna, AstraZeneca, AbbVie and Ely Lilly, paid to his institution. H.F.G. has received research grants from the Swiss National Science Foundation, Swiss HIV Cohort Study, Yvonne Jacob Foundation, NIH, Gilead, ViiV and Bill and Melinda Gates foundation, paid to his institution; personal honoraria for data safety monitoring board or advisory board consultations from Merck, ViiV healthcare, Gilead Sciences, Janssen, Johnson and Johnson, Novartis and GSK; and personal travel expenses from Gilead. J.N. received research grants from the Swiss HIV Cohort Study and the cantonal hospital St. Gallen, paid to her institution, and travel expenses from Gilead. K.J.M. received unrestricted research grants from Gilead and Novartis, paid to her institution, and personal honoraria for advisory board consultations from ViiV. M.C. received research grants from Gilead, ViiV and MSD, paid to his institution; payment for expert testimony from Gilead, ViiV and MSD, paid to his institution; and travel expenses from Gilead, paid to his institution. M.S. received honoraria for data safety monitoring board advisory board consultations from Gilead, ViiV, Moderna, Pfizer and MSD, paid to his institution, and travel expenses for conferences from Gilead, paid to his institution. P.F. received personal travel expenses from the University Zurich, payment for equipment from the University Zurich and personal honoraria for presentations from the University of Zurich. R.D.K. received research grants from Gilead and NIH, paid to his institution. All other authors report no potential conflicts.
Figures
Similar articles
-
Genetic attributes of blood-derived subtype-C HIV-1 tat and env in India and neurocognitive function.J Med Virol. 2014 Jan;86(1):88-96. doi: 10.1002/jmv.23816. Epub 2013 Oct 22. J Med Virol. 2014. PMID: 24150902 Free PMC article.
-
Comparative HIV-1 Phylogenies Characterized by PR/RT, Pol and Near-Full-Length Genome Sequences.Viruses. 2022 Oct 17;14(10):2286. doi: 10.3390/v14102286. Viruses. 2022. PMID: 36298841 Free PMC article.
-
The association between depressive symptoms and neurocognitive impairment in people with well-treated HIV in Switzerland.Int J STD AIDS. 2021 Jul;32(8):729-739. doi: 10.1177/0956462420987434. Epub 2021 Feb 25. Int J STD AIDS. 2021. PMID: 33629882
-
Anticholinergic medication use in elderly people living with HIV and self-reported neurocognitive impairment: a prospective cohort study.J Antimicrob Chemother. 2022 Feb 2;77(2):492-499. doi: 10.1093/jac/dkab386. J Antimicrob Chemother. 2022. PMID: 34734255 Review.
-
Is There Any Evidence of Premature, Accentuated and Accelerated Aging Effects on Neurocognition in People Living with HIV? A Systematic Review.AIDS Behav. 2021 Mar;25(3):917-960. doi: 10.1007/s10461-020-03053-3. Epub 2020 Oct 6. AIDS Behav. 2021. PMID: 33025390 Free PMC article.
Cited by
-
Phylogenetics and molecular evolution to understand and curb the HIV pandemic.Nat Rev Microbiol. 2025 Jun 30. doi: 10.1038/s41579-025-01202-w. Online ahead of print. Nat Rev Microbiol. 2025. PMID: 40588586 Review.
-
Addressing data management and analysis challenges in viral genomics: The Swiss HIV cohort study viral next generation sequencing database.PLOS Digit Health. 2025 Apr 21;4(4):e0000825. doi: 10.1371/journal.pdig.0000825. eCollection 2025 Apr. PLOS Digit Health. 2025. PMID: 40257980 Free PMC article.
References
-
- Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24(9):1243–1250. - PubMed
LinkOut - more resources
Full Text Sources
