Network pharmacology- and cell-based assessments identify the FAK/Src pathway as a molecular target for the antimetastatic effect of momordin Ic against cholangiocarcinoma
- PMID: 38961933
- PMCID: PMC11219314
- DOI: 10.1016/j.heliyon.2024.e32352
Network pharmacology- and cell-based assessments identify the FAK/Src pathway as a molecular target for the antimetastatic effect of momordin Ic against cholangiocarcinoma
Abstract
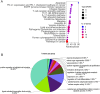
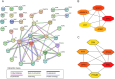
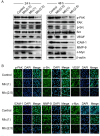
Previous studies have indicated the efficacy of momordin Ic (MIc), a plant-derived triterpenoid, against several types of cancers, implying its potential for further development. However, comprehensive insights into the molecular mechanisms and targets of MIc in cholangiocarcinoma (CCA) are lacking. This study aimed to investigate the actions of MIc against CCA at the molecular level. Network pharmacology analysis was first employed to predict the mechanisms and targets of MIc. The results unveiled the potential involvement of MIc in apoptosis and cell migration, pinpointing Src and FAK as key targets. Subsequently, cell-based assays, in accordance with FAK/Src-associated metastasis, were conducted, demonstrating the ability of MIc to attenuate the metastatic behaviours of KKU-452 cells. The in vitro results further indicated the capability of MIc to suppress the epithelial-mesenchymal transition (EMT) process, notably by downregulating EMT regulators, including N-cadherin, vimentin, ZEB2 and FOXC1/2 expression. Furthermore, MIc suppressed the activation of the FAK/Src signalling pathway, influencing critical downstream factors such as MMP-9, VEGF, ICAM-1, and c-Myc. Molecular docking simulations also suggested that MIc could interact with FAK and Src domains and restrain kinases from being activated by hindering ATP binding. In conclusion, this study employs a comprehensive approach encompassing network pharmacology analysis, in vitro assays, and molecular docking to unveil the mechanisms and targets of MIc in CCA. MIc mitigates metastatic behaviours and suppresses key pathways, offering a promising avenue for future therapeutic strategies against this aggressive cancer.
Keywords: Antimetastasis; Cholangiocarcinoma; FAK; Momordin Ic; Network pharmacology; Src.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Anti-metastatic Potential of Natural Triterpenoid Cucurbitacin B Against Cholangiocarcinoma Cells by Targeting Src Protein.Integr Cancer Ther. 2022 Jan-Dec;21:15347354221124861. doi: 10.1177/15347354221124861. Integr Cancer Ther. 2022. PMID: 36154723 Free PMC article.
-
Cucurbitacin B Diminishes Metastatic Behavior of Cholangiocarcinoma Cells by Suppressing Focal Adhesion Kinase.Asian Pac J Cancer Prev. 2021 Jan 1;22(1):219-225. doi: 10.31557/APJCP.2021.22.1.219. Asian Pac J Cancer Prev. 2021. PMID: 33507702 Free PMC article.
-
Epidermal growth factor receptor as a potential target of momordin Ic to promote apoptosis of cholangiocarcinoma cells.J Pharm Pharmacol. 2022 Jul 15;74(7):996-1005. doi: 10.1093/jpp/rgac033. J Pharm Pharmacol. 2022. PMID: 35640567
-
Activation of Vimentin Is Critical to Promote a Metastatic Potential of Cholangiocarcinoma Cells.Oncol Res. 2018 May 7;26(4):605-616. doi: 10.3727/096504017X15009778205068. Epub 2017 Jul 25. Oncol Res. 2018. PMID: 28762325 Free PMC article.
-
The novel epithelial-mesenchymal transition-related proteins and their therapeutic targets in cholangiocarcinoma: a narrative review.J Gastrointest Oncol. 2023 Jun 30;14(3):1593-1612. doi: 10.21037/jgo-22-1126. Epub 2023 May 26. J Gastrointest Oncol. 2023. PMID: 37435208 Free PMC article. Review.
References
-
- Banales J.M., Cardinale V., Carpino G., et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nat. Rev. Gastroenterol. Hepatol. 2016;13:261–280. doi: 10.1038/nrgastro.2016.51. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

