Physical analysis of aspirin in different phases and states using density functional theory
- PMID: 38961960
- PMCID: PMC11219974
- DOI: 10.1016/j.heliyon.2024.e32610
Physical analysis of aspirin in different phases and states using density functional theory
Abstract
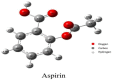
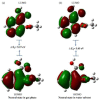
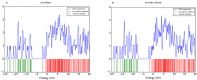
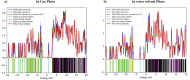
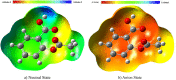
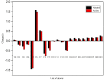
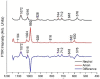
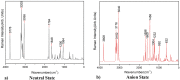
This study analyzed the aspirin molecule (C9H8O4) using Density Functional Theory (DFT) on Gaussian 09W software. First, the structure of aspirin was optimized using the DFT method with the B3LYP functional and the 6-311+G (d,p) basis set. A global reactivity study was employed to understand the reactivity of aspirin in gas and solvent water for both anion and neutral states. To understand the involvement of orbitals in chemical stability and electron conductivity, we calculated the HOMO-LUMO. The thermodynamic function of a molecule was understood using thermochemistry. Molecular Electrostatic Potential (MEP) was employed to understand the physiochemical properties of aspirin. We observed the Mulliken atomic charge to calculate the atomic charge of aspirin. Finally, the title molecule's UV-Vis, FTIR, and Raman spectra are analyzed and compared with the experimental data.
Keywords: DOS; HOMO AND LUMO; MEP; Mulliken atomic charge; Spectroscopy.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Spectroscopic, quantum chemical, and topological calculations of the phenylephrine molecule using density functional theory.Sci Rep. 2025 Jan 2;15(1):208. doi: 10.1038/s41598-024-81633-2. Sci Rep. 2025. PMID: 39747169 Free PMC article.
-
Molecular simulation, vibrational spectroscopy and global reactivity descriptors of pseudoephedrine molecule in different phases and states.Heliyon. 2023 Mar 21;9(3):e14801. doi: 10.1016/j.heliyon.2023.e14801. eCollection 2023 Mar. Heliyon. 2023. PMID: 37101481 Free PMC article.
-
FTIR, FT-RAMAN, NMR, spectra, normal co-ordinate analysis, NBO, NLO and DFT calculation of N,N-diethyl-4-methylpiperazine-1-carboxamide molecule.Spectrochim Acta A Mol Biomol Spectrosc. 2013 Nov;115:275-86. doi: 10.1016/j.saa.2013.06.011. Epub 2013 Jun 20. Spectrochim Acta A Mol Biomol Spectrosc. 2013. PMID: 23845985
-
Spectroscopic (infrared, Raman, UV and NMR) analysis, Gaussian hybrid computational investigation (MEP maps/HOMO and LUMO) on cyclohexanone oxime.Spectrochim Acta A Mol Biomol Spectrosc. 2012 Oct;96:207-20. doi: 10.1016/j.saa.2012.03.090. Epub 2012 May 14. Spectrochim Acta A Mol Biomol Spectrosc. 2012. PMID: 22683556
-
Quantum mechanical study and spectroscopic (FT-IR, FT-Raman, UV-Visible) study, potential energy surface scan, Fukui function analysis and HOMO-LUMO analysis of 3-tert-butyl-4-methoxyphenol by DFT methods.Spectrochim Acta A Mol Biomol Spectrosc. 2014 Sep 15;130:604-20. doi: 10.1016/j.saa.2014.04.058. Epub 2014 Apr 20. Spectrochim Acta A Mol Biomol Spectrosc. 2014. PMID: 24813291
Cited by
-
Spectroscopic, quantum chemical, and topological calculations of the phenylephrine molecule using density functional theory.Sci Rep. 2025 Jan 2;15(1):208. doi: 10.1038/s41598-024-81633-2. Sci Rep. 2025. PMID: 39747169 Free PMC article.
-
Aspirin in Cancer Therapy: Pharmacology and Nanotechnology Advances.Int J Nanomedicine. 2025 Feb 23;20:2327-2365. doi: 10.2147/IJN.S505636. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40017626 Free PMC article. Review.
-
Quantum physical analysis of caffeine and nicotine in CCL4 and DMSO solvent using density functional theory.Sci Rep. 2025 Mar 26;15(1):10372. doi: 10.1038/s41598-025-91211-9. Sci Rep. 2025. PMID: 40140665 Free PMC article.
References
-
- Patrono C. Aspirin as an antiplatelet drug. N. Engl. J. Med. 1994;330(18):1287–1294. https://doi:10.1056/NEJM199405053301808 - DOI - PubMed
LinkOut - more resources
Full Text Sources

